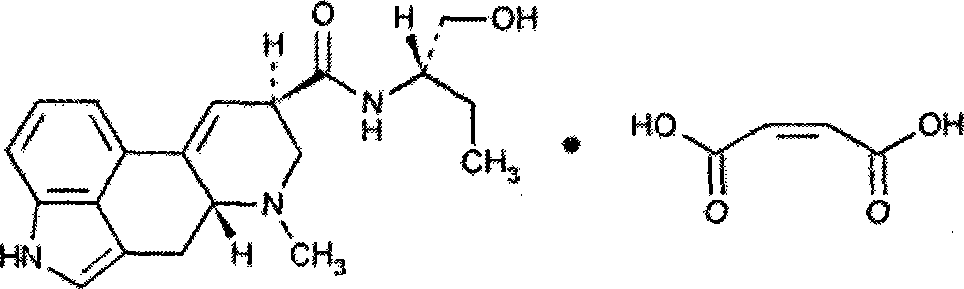
Methylergometrine Maleate powder injection and preparation method thereof
A technology of methysergide maleate and maleic acid, which is applied in powder delivery, freeze-drying delivery, diseases, etc., can solve problems such as poor stability of aqueous injections, drug decomposition and destruction, light and heat instability, etc., and achieve Effects of reducing irritation and increasing compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] The following examples and experimental data of influencing factors are used to further illustrate the technical solution of the present invention, but not to limit the technical characteristics of the present invention.
[0018] The specific process and steps of preparing the freeze-dried powder injection of the present invention are as follows:
[0019] (1) Dosing
[0020] Preparation: all kinds of liquid preparation supplies are ready;
[0021] Dissolving excipients: weigh the prescribed amount of pharmaceutically acceptable carrier, add appropriate amount of sterilized water for injection, dissolve at room temperature, add appropriate amount of activated carbon for needles, stir at room temperature for 30 minutes, and coarsely filter;
[0022] Dissolving the main drug: take the prescribed amount of methysergide maleate, add appropriate amount of water to dissolve, add to the above crude filtrate, add water to the full amount of the prescription;
[0023] Steriliza...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com