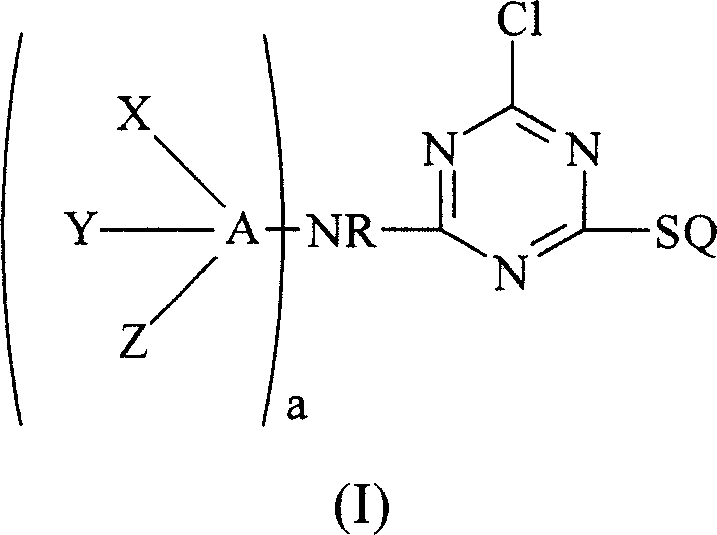
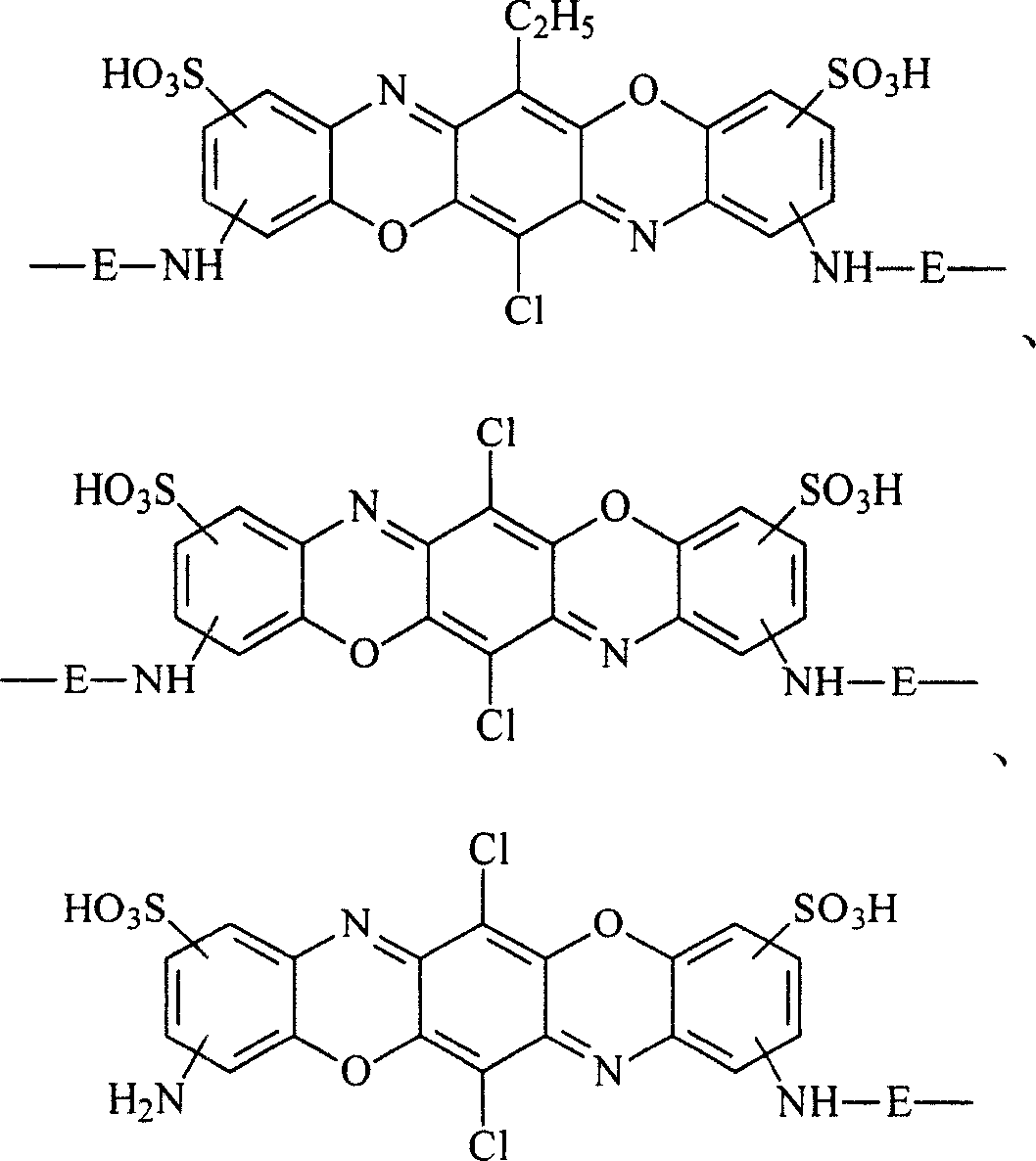
Chemically-reactive dyes with thioalkyl-s-triazine reactive group
A reactive dye, triazine technology, applied in reactive dyes, azo dyes, organic dyes, etc., can solve the problems of low economic benefit of dye products and high production cost of trifluoro-s-triazine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] (a) Take 19.45 parts of cyanuric chloride and disperse in 150 parts of water at 0°C, then add 31.9 parts of 2-amino-5-naphthol-1,7-disulfonic acid (2-Amino-5 -hydroxy-naphthalene-1,7-disulfonic acid) powder, with 15% sodium carbonate (Na 2 CO 3 ) aqueous solution to adjust the pH value of the reaction solution to 3, naturally raise the temperature to 20° C. and keep the temperature for 1 to 2 hours for later use.
[0068] (b) Add 9.7 parts of Thioglycolic acid to the aqueous solution obtained in (a) above, add 18 parts of sodium carbonate (Na 2 CO 3 ) powder to adjust the pH value of the reaction solution to between 7 and 7.5, and after maintaining for 15 minutes, adjust the pH value of the reaction solution to 6 to 6.5 with hydrochloric acid (HCl) aqueous solution, and through commonly used sodium chloride (NaCl) salting out and filtration, Take the filter cake and set aside.
[0069] (c) Take 150 parts of water at 0°C and add 29.5 parts of 1-aminobenzene-4-(β-viny...
Embodiment 2
[0072] (a) Disperse 19.45 parts of cyanuric chloride in 150 parts of water at 0°C, add 31.5 parts of 1-naphthol-8-amino-3,6-disulfonic acid (1-Naphthol-8-amino-3, 6-disulfonic acid) powder, adjust the pH value of the reaction solution to 3 with 15% sodium carbonate aqueous solution, naturally raise the temperature to 20°C and keep the temperature for 1-2 hours for later use.
[0073] (b) Add 9.7 parts of thioglycolic acid to the aqueous solution obtained in (a) above, adjust the pH value of the reaction solution to 7 to 7.5 with 18 parts of sodium carbonate at 20°C, and adjust the pH of the mixed solution with aqueous hydrochloric acid after keeping for 15 minutes The value is 6 to 6.5. After the commonly used sodium chloride salting out and filtration, the filter cake is taken for later use.
[0074] (c) Take 150 parts of water at 0°C, add 29 parts of p-Aminosulfoethylsulfate (p-Aminosulfoethylsulfate) and 50 parts of 32% hydrochloric acid aqueous solution to fully stir and d...
Embodiment 3
[0077] (a) Disperse 19.45 parts of cyanuric chloride in 150 parts of water at 0°C, add 18.8 parts of 1,3-phenylenediamine (1,3-Phenylenediamine) powder, and adjust the pH value of the reaction solution with 15% aqueous sodium carbonate solution 3, naturally warm up to 20°C and keep the temperature for 1-2 hours for later use.
[0078] (b) Add 9.7 parts of thioglycolic acid to the aqueous solution obtained in (a) above, adjust the pH of the reaction solution to 7 to 7.5 with 18 parts of sodium carbonate at 20°C, and adjust the pH of the reaction solution with aqueous hydrochloric acid after keeping for 15 minutes The value is 6 to 6.5. After the commonly used sodium chloride salting out and filtration, the filter cake is taken for later use.
[0079] (c) Take 150 parts of water at 0°C, add 19.5 parts of the filter cake obtained in the above (b) and 25 parts of 32% hydrochloric acid aqueous solution to fully stir and disperse, then quickly add 3.6 parts of sodium nitrite aqueous...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com