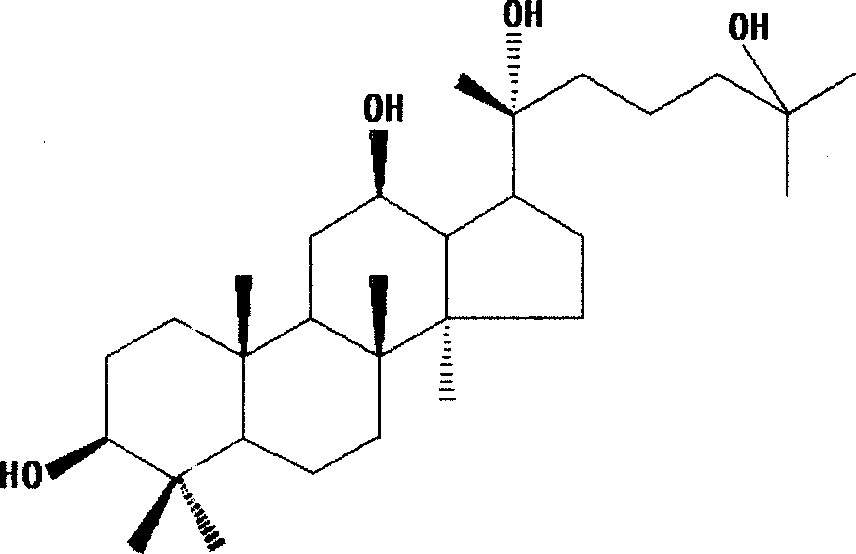
Method for preparing 20(R)-25-hydroxy-dammarane type-3beta,12beta,20-triol
A dammarane-type, hydroxyl-based technology, applied in the direction of preparation of steroids, methods based on microorganisms, biochemical equipment and methods, etc., can solve problems such as difficulties in obtaining large quantities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Preparation of 20(R)-25-OH-PPD by silica gel chromatography
[0018] After the fresh ginseng fruit of Araliaceae Panax plant of 5kg drying is extracted with 70% ethanol, after passing through the D101 macroporous adsorption resin column (provided by Tianjin Chemical Co., Ltd.), after washing, use 70% ethanol to elute from the column to separate and purify it and drying to obtain total saponins of ginseng fruit. Take 10 g of total saponins of ginseng fruit, extract with chloroform, carry out silica gel column chromatography to separate the chloroform extract, and obtain 7 fractions by chloroform:methanol (30:1-5:1) gradient elution, and fraction 5 is passed through the reverse phase silica gel column layer After separation, acetonitrile: water (80:20) elution and ethyl acetate recrystallization, 20(R)-25-OH-PPD was obtained with a yield of 0.18%.
Embodiment 2
[0019] Example 2: Preparation of 20(R)-25-OH-PPD by alkaline hydrolysis
[0020] Weigh 10 g of total ginsenosides, dissolve in 1000 ml of 95% ethanol solution, filter, remove insoluble matter, and obtain a filtrate. Add the prepared 0.5% (W / V) sodium hydroxide ethanol solution into the above filtrate under stirring, let it stand still, filter, and wash the precipitate with water until neutral. The dried precipitate was separated by silica gel column chromatography, and 8 fractions were obtained by gradient elution of petroleum: ethyl acetate (30:1-1:1), and fraction 5 was recrystallized from ethyl acetate to obtain 20(R) -25-OH-PPD white crystal, yield 1.2%.
Embodiment 3
[0021] Example 3: Preparation of 20(R)-25-OH-PPD by hydrolysis of sodium hydroxide
[0022] Weigh 10 g of total saponins of ginseng fruit, dissolve in 1000 ml of 2.5 mol / L sodium hydroxide and 80% methanol aqueous solution and hydrolyze for 24 hours, neutralize the reaction solution with 2.5 mol / L hydrochloric acid, recover methanol under reduced pressure, and extract with chloroform The reaction solution and the chloroform phase were washed with water, dried over anhydrous sodium sulfate, and evaporated to dryness to collect the residue, which was separated by silica gel column chromatography and gradient eluted with petroleum: ethyl acetate (50:1-2:1) to obtain 8 fractions. Fraction 5 was recrystallized from ethyl acetate to obtain white crystals of 20(R)-25-OH-PPD with a yield of 1.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com