Diamine, liquid crystal tropism agent, liquid crystal tropism film and liquid crystal display device
A technology of diamine and tetracarboxylic dianhydride, used in liquid crystal materials, chemical instruments and methods, instruments, etc., can solve problems such as narrow viewing angle, decreased brightness or contrast, and mid-tone brightness conversion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0486] A mixture of 10g (38mmol) of 4-(4-pentylcyclohexyl)cyclohexanecarboxaldehyde, 12g (92mmol) of aniline hydrochloride and 11g (114mmol) of aniline synthesized according to the method described in JP 2002-121190 Stir at 160°C for 12 hours. After cooling, the reaction solution was poured into a 20% sodium hydroxide aqueous solution (300 ml), and extraction was performed with dichloromethane (300 ml). After the organic layer was washed twice with pure water (300 ml), anhydrous magnesium sulfate was added and dried. After the magnesium sulfate was filtered, the solvent was distilled off under reduced pressure. It was separated and purified by column chromatography (dichloromethane:methanol=20:1), and the obtained crude crystals were recrystallized from ethanol to obtain 1,1-bis(4-aminophenyl)-1-(4-(4) -Pentylcyclohexyl)cyclohexyl)methane (5HHDDM). The yield is 4.0 g, and the yield is 24%.
[0487] Melting point: 182.6-185.8°C
[0488] 1H-NM R: 0.81-1.29 (m, 21H), 1.63-1.66 (m, 9H...
Embodiment 2
[0490] Using 34 g (121 mmol) of 4-dodecylcyclohexanecarboxaldehyde synthesized according to the method described in JP 2002-121190, the reaction and post-treatment were carried out in the same manner as in Synthesis Example 1 above. It was separated and purified by column chromatography (dichloromethane:methanol=10:1), and the obtained crude crystals were recrystallized from ethanol twice to obtain 1,1-bis(4-aminophenyl)-1-(4- Dodecylcyclohexyl) methane (12HDDM). The yield is 7.8g, and the yield is 14%.
[0491] Melting point: 104.6-107.3°C
[0492] 1H-NM R: 0.86-1.26 (m, 27H), 1.58-1.66 (m, 7H), 1.92 (d, J=10.00, 1H), 2.22 (d, J=10.00, 1H), 3.60 (br.S , 4H), 6.50-6.66 (m, 4H), 7.11-7.23 (m, 4H)
Embodiment 3
[0494] Using 28 g (85 mmol) of 4-(n-hexadecyl)benzaldehyde synthesized according to the method described in JP 2002-121190 A, the reaction and post-treatment were performed in the same manner as in Synthesis Example 1 above. Separate and purify by column chromatography (toluene:methanol=10:1), and recrystallize the obtained crude crystals from ethanol twice to obtain bis(4-aminophenyl)-(4-(n-hexadecyl)benzene Base) methane (16PDDM). The yield is 17g, and the yield is 41%.
[0495] Melting point: 90.9-92.8°C
[0496] 1H-NM R; 0.88 (t, 3H, J = 6.95), 1.25-1.30 (m, 28H), 2.55 (t, 2H, J = 8.15), 3.56 (br.s, 4H), 5.30 (s, 1H) ), 6.60 (d, 4H, J = 8.30), 6.89 (d, 4H, J = 8.20), 6.94-7.26 (m, 4H)
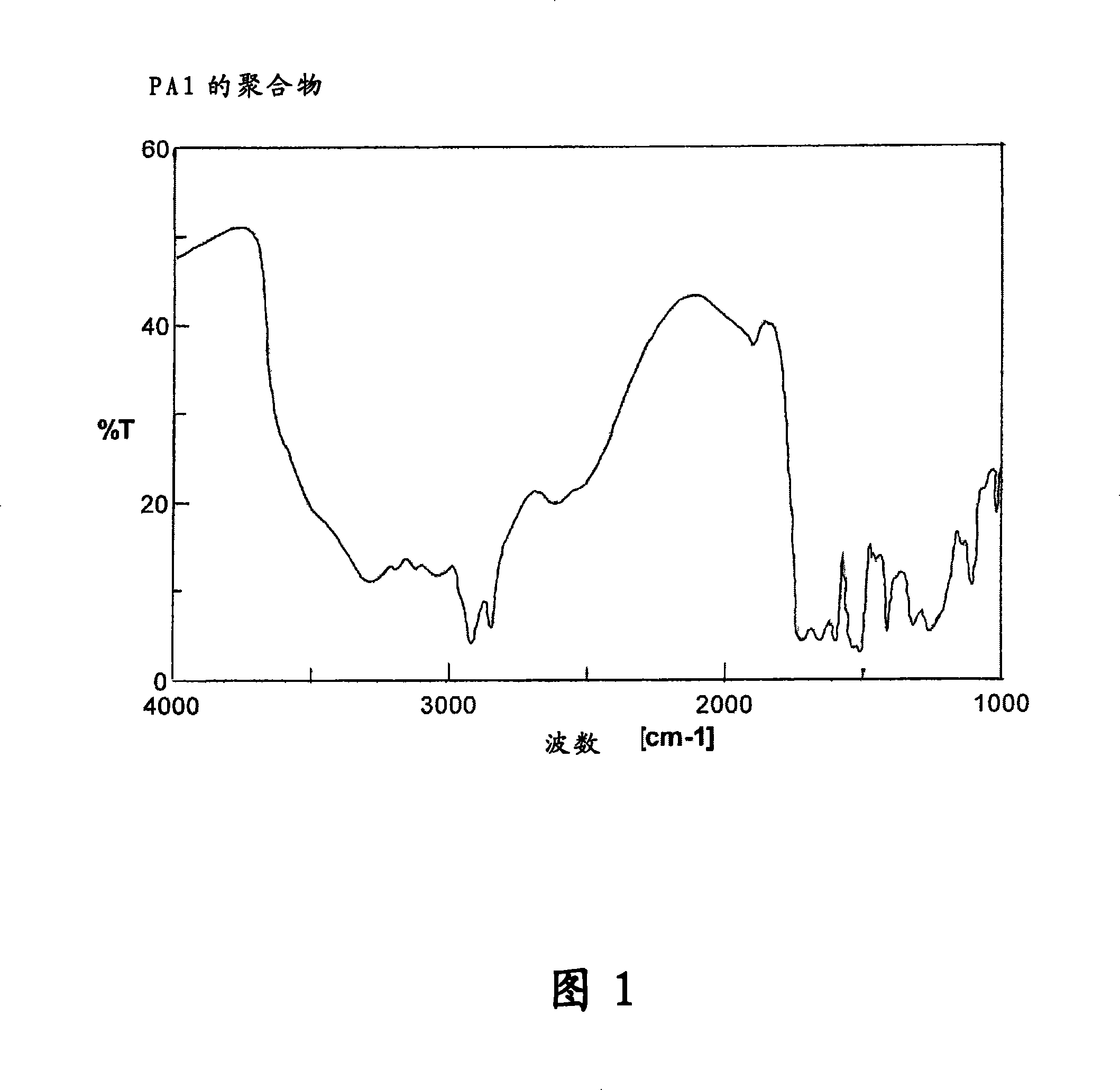
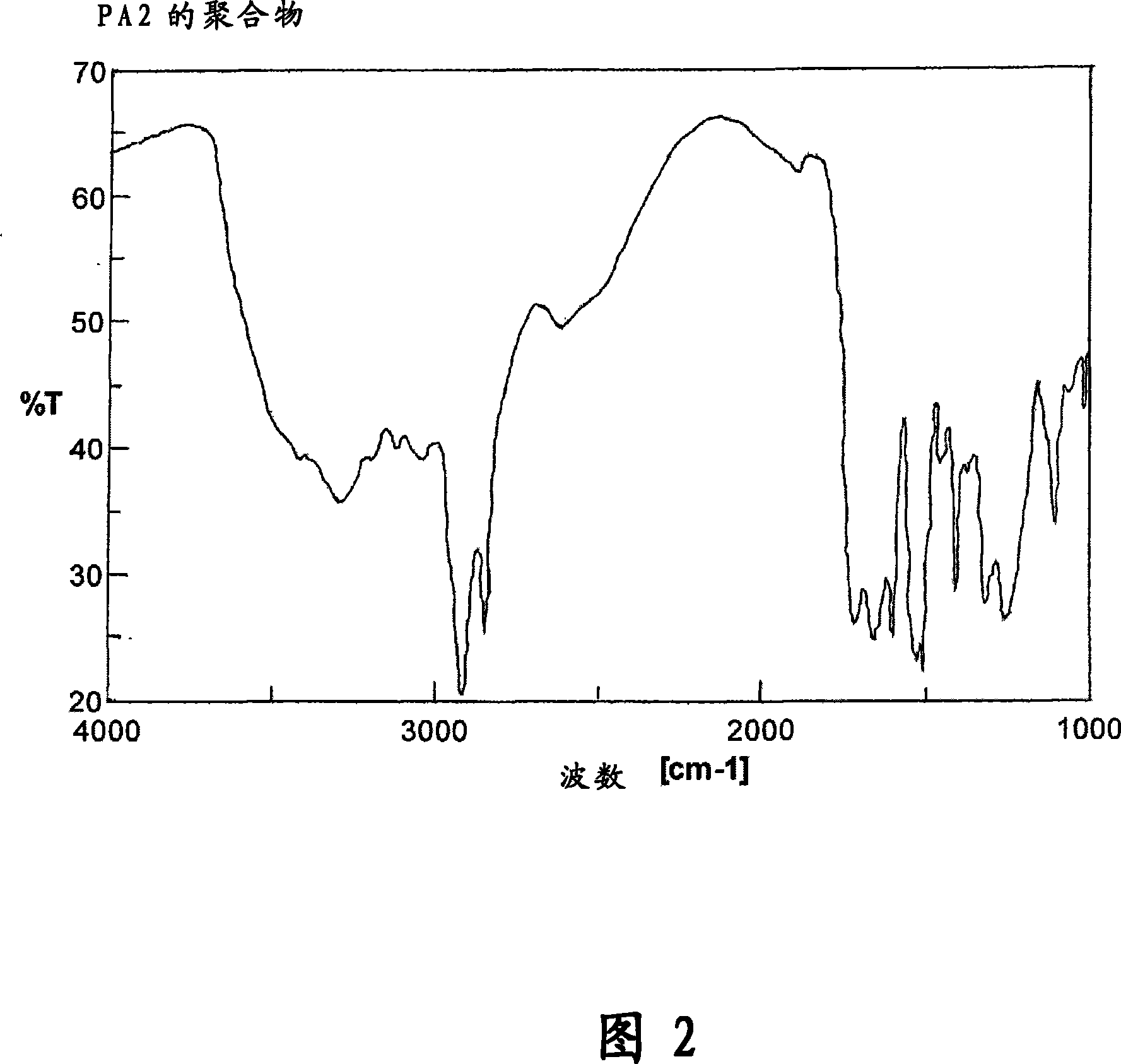
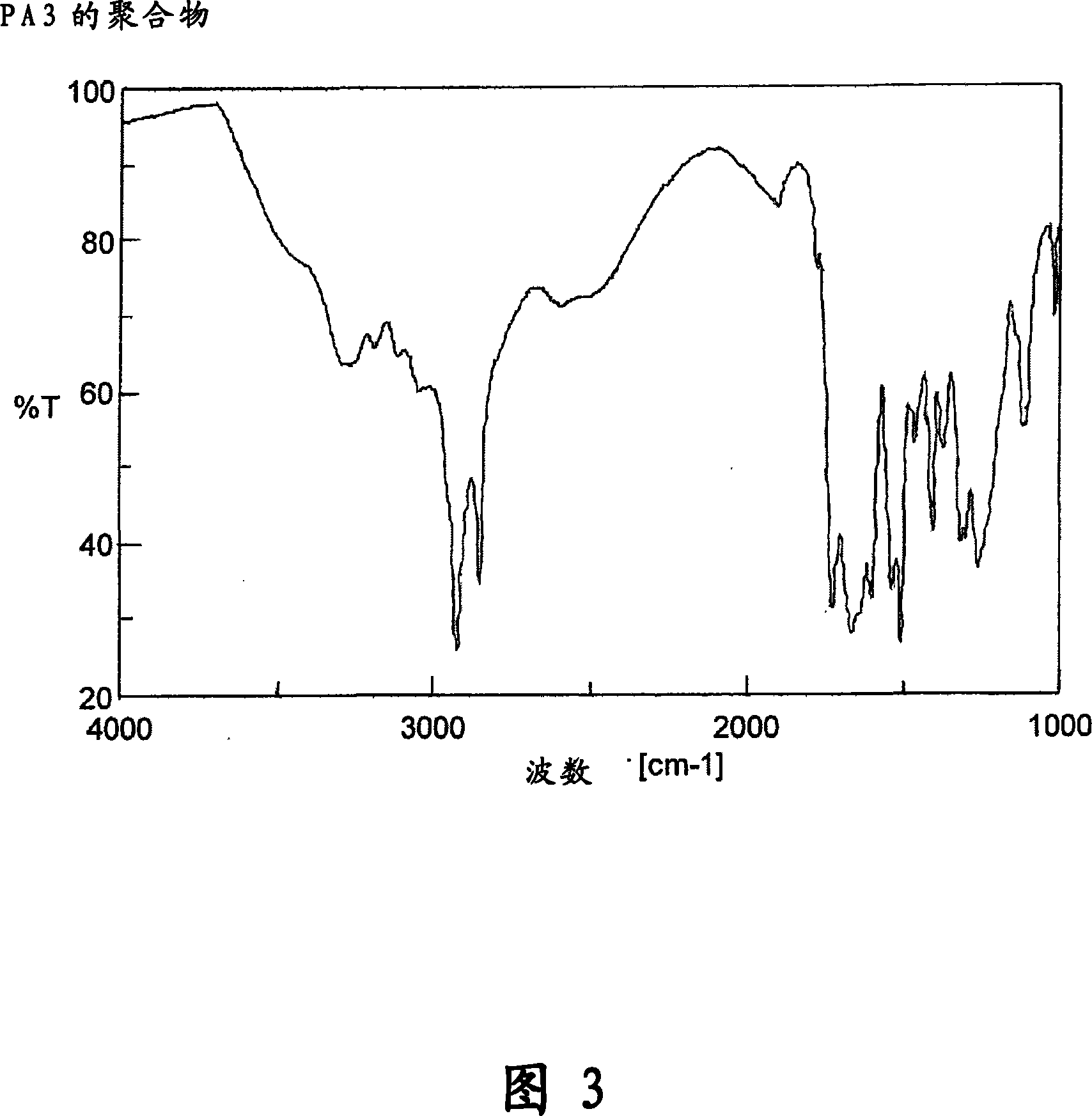
[0497]
[0498] [Synthesis of polyamic acid]
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com