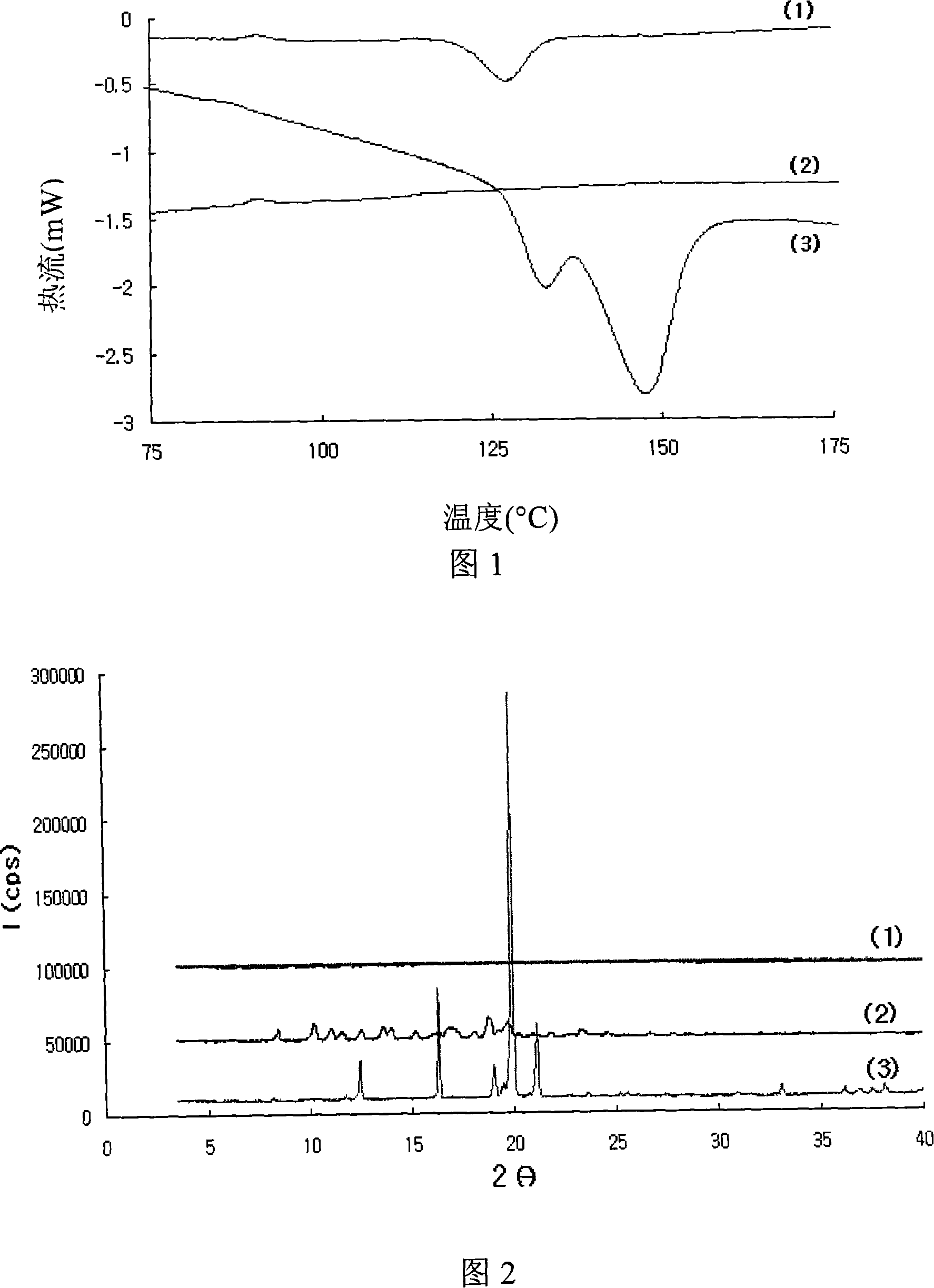
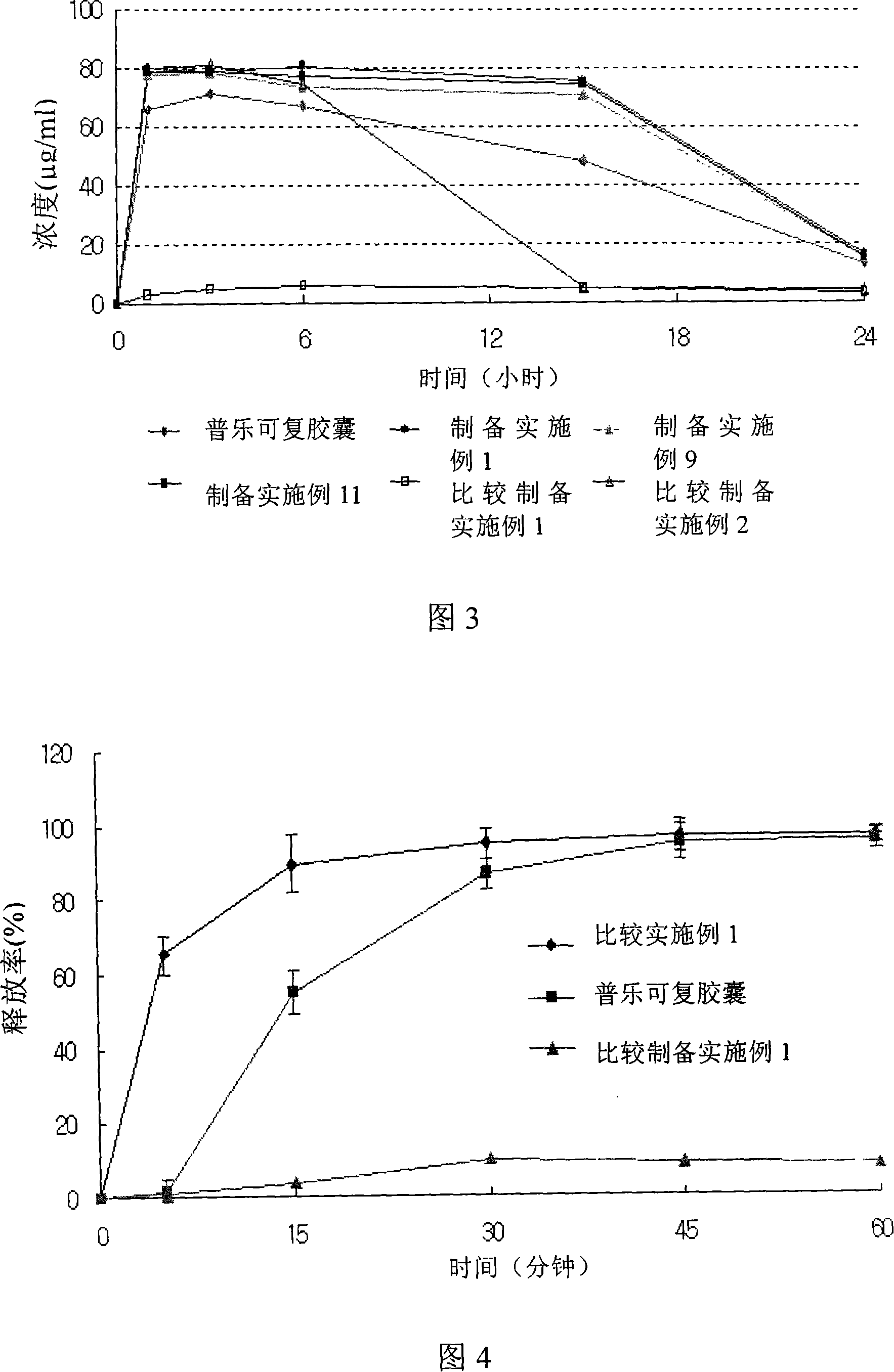
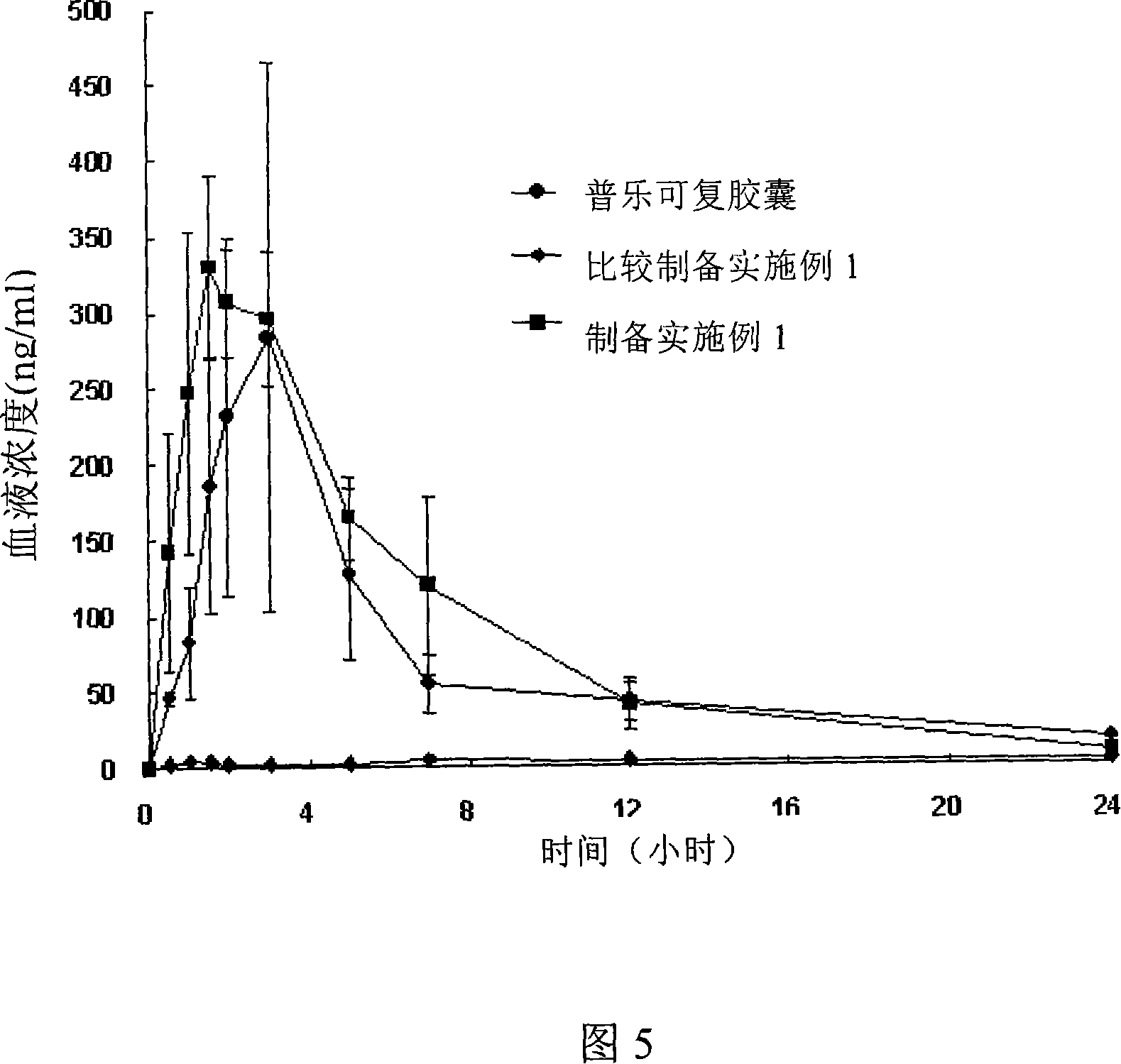
Amorphous taclolimus solid dispersion having an enhanced solubility and pharmaceutical composition comprising same
A technology of solid dispersion and tacrolimus, which can be used in pharmaceutical combinations, organic active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] 400 mg of 2-hydroxypropyl-β-cyclodextrin (Aldrich, USA), 20 mg of citric acid and 20 mg of Poloxamer (Poloxamer) 188 (Lutrol F68, BASF) were added to MC (dichloromethane) and ethanol and stirred until the solution became clear. Then, 400 mg of lactose was added thereto and dispersed evenly. 100 mg of tacrolimus dissolved in ethanol was mixed with the dispersion and spray-dried while the inlet and outlet temperatures of the spray dryer (mini spray dryer B-191, Buchi, Switzerland) were maintained at 60 °C, respectively. °C and 45-50 °C to obtain a solid dispersion of tacrolimus with the ingredients listed in Table 1.
Embodiment 2
[0065] A tacrolimus solid dispersion having the ingredients listed in Table 1 was prepared by repeating the procedure of Example 1 except that 200 mg of 2-hydroxypropyl-β-cyclodextrin was used.
Embodiment 3
[0067] A tacrolimus solid dispersion having the ingredients listed in Table 1 was prepared by repeating the procedure of Example 1 except that 800 mg of 2-hydroxypropyl-β-cyclodextrin was used.
[0068] Table 1
[0069]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com