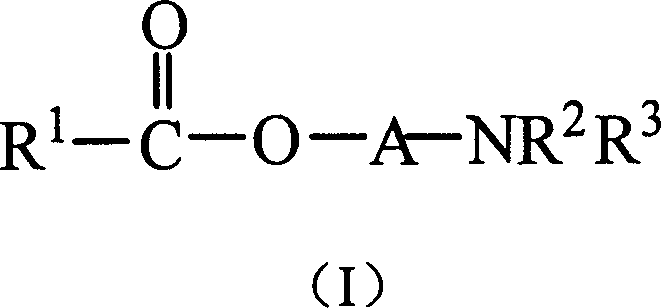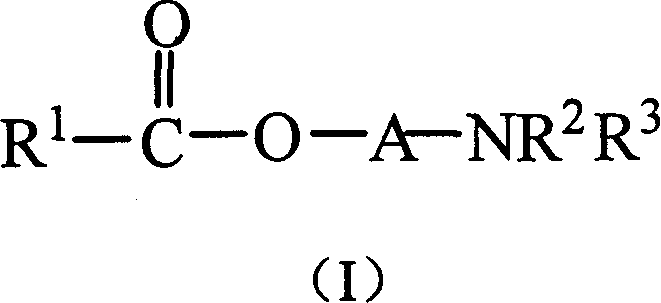Catalyst component used for olefin polymerization reaction and its catalyst
A technology for olefin polymerization and catalyst, applied in the field of solid catalyst components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1 Synthesis of 1-benzamido-3-benzoyloxybutane
[0075] Add 1-amino-3-butanol (5.2g), sodium bicarbonate (6.4g), tetrahydrofuran (50ml) into a reaction flask equipped with a stirring device and a reflux condenser, add benzoyl chloride (7ml) dropwise under stirring ) and tetrahydrofuran (30ml). After the addition, react at room temperature for 1 hour, then heat to reflux for 4 hours. After the reaction was completed, the temperature was lowered, diluted with water, extracted three times with ether, and the organic phases were combined. Wash with water at 40°C and then with saturated sodium chloride solution, and dry the organic phase over anhydrous magnesium sulfate overnight. The product was obtained by column chromatography after removing the solvent.
[0076] 1 H-NMR (δ, ppm, TMS, CDCl 3 ): 7.3~8.0 (10H, m, ArH), 4.0 (1H, m, OCH), 2.5~2.7 (2H, m, NCH 2 ), 1.2 (2H, m, CH 3 ), 0.9 (3H, d, CH 3 ).
Embodiment 2
[0077] Example 2 Synthesis of 1-benzoyloxy-3-benzamidopropane
[0078] Add 3-aminopropanol (4.6g), sodium bicarbonate (9.6g), tetrahydrofuran (50ml) into a reaction flask equipped with a stirring device and a reflux condenser, and add benzoyl chloride (14ml) and tetrahydrofuran dropwise under stirring (30ml). After the addition, react at room temperature for 1 hour, then heat to reflux for 4 hours. After the reaction was completed, the temperature was lowered, diluted with water, extracted three times with ether, and the organic phases were combined. Wash with water at 40°C and then with saturated sodium chloride solution, and dry the organic phase over anhydrous magnesium sulfate overnight. The product was obtained by column chromatography after removing the solvent.
[0079] 1 H-NMR (δ, ppm, TMS, CDCl 3 ): 7.3~8.1 (10H, m, ArH), 3.9 (2H, t, OCH 2 ), 2.4~2.6 (2H, m, NCH 2 ), 1.0 (2H, m, CH 2 ).
Embodiment 3
[0080] Example 3 Synthesis of 1-dimethylamino-2-benzoyloxypropane
[0081] Add 1-dimethylamino-2-propanol (5.7g), sodium bicarbonate (6.4g), tetrahydrofuran (50ml) in a reaction flask equipped with a stirring device and a reflux condenser, add benzoyl chloride ( 7ml) and tetrahydrofuran (25ml). After the addition, react at room temperature for 1 hour, then heat to reflux for 4 hours. After the reaction was completed, the temperature was lowered, diluted with water, extracted three times with ether, and the organic phases were combined. Wash with water at 40°C and then with saturated sodium chloride solution, and dry the organic phase over anhydrous magnesium sulfate overnight. The product was obtained by column chromatography after removing the solvent.
[0082] 1 H-NMR (δ, ppm, TMS, CDCl 3 ): 7.3~8.0 (5H, m, ArH), 4.3 (1H, m, OCH), 2.8~2.9 (2H, m, NCH 2 ), 2.4 (6H, s, CH 3 ), 0.9 (3H, t, CH 3 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melt index | aaaaa | aaaaa |
| Melt index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com