Hypoglycemic medicine composition
A technology of composition and medicine, which is applied in the field of medicine, can solve the problems that there is no research report on B vitamins of sulfonylurea hypoglycemic drugs, and that the harm of Hcy to diabetic patients cannot be alleviated.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Example 1 Preparation of compound glimepiride rosiglitazone folic acid (0.4mg) tablets (quantity of 1000 tablets)
[0132] Formula: Glimepiride 1g
[0133] Rosiglitazone 4g
[0134] Folic acid 0.4g
[0135] Starch 20g
[0136] Microcrystalline Cellulose 40g
[0137] Sodium carboxymethyl starch 40g
[0138] Low-substituted hydroxypropyl cellulose 20g
[0139] Proper amount of povidone ethanol solution
[0140] Magnesium Stearate 0.5%
[0141] Preparation method: crush the auxiliary materials through an 80-mesh sieve, and dry for later use. Take 1g of glimepiride, 4g of rosiglitazone, and 0.4g of folic acid and mix them uniformly according to the method of equal increase, and then add starch, microcrystalline cellulose, sodium carboxymethyl starch and low-substituted hydroxypropyl cellulose according to the prescription amount, Mix evenly according to equal increment method, use 5% povidone K29 / 30-95% ethanol soluti...
Embodiment 2~ Embodiment 8
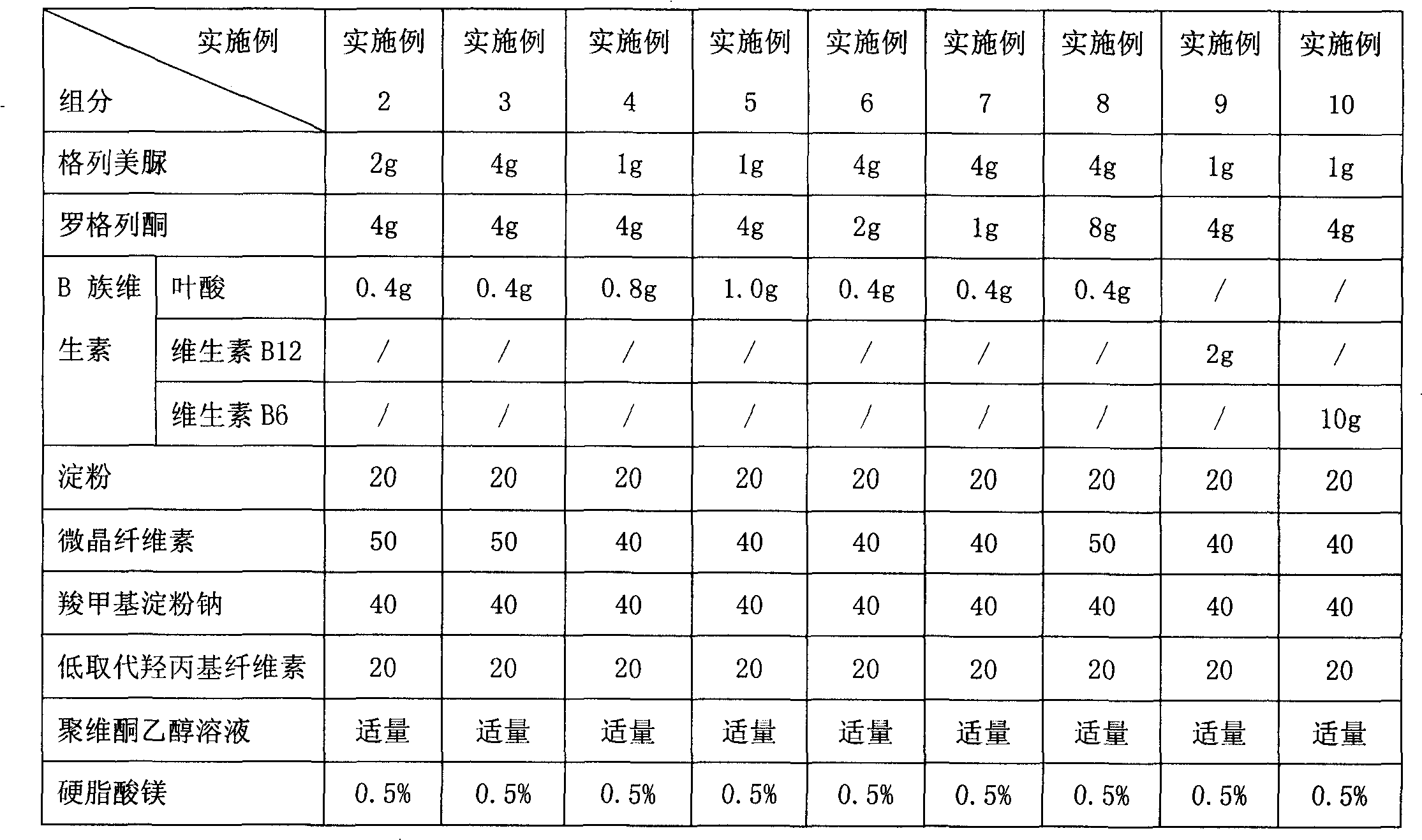
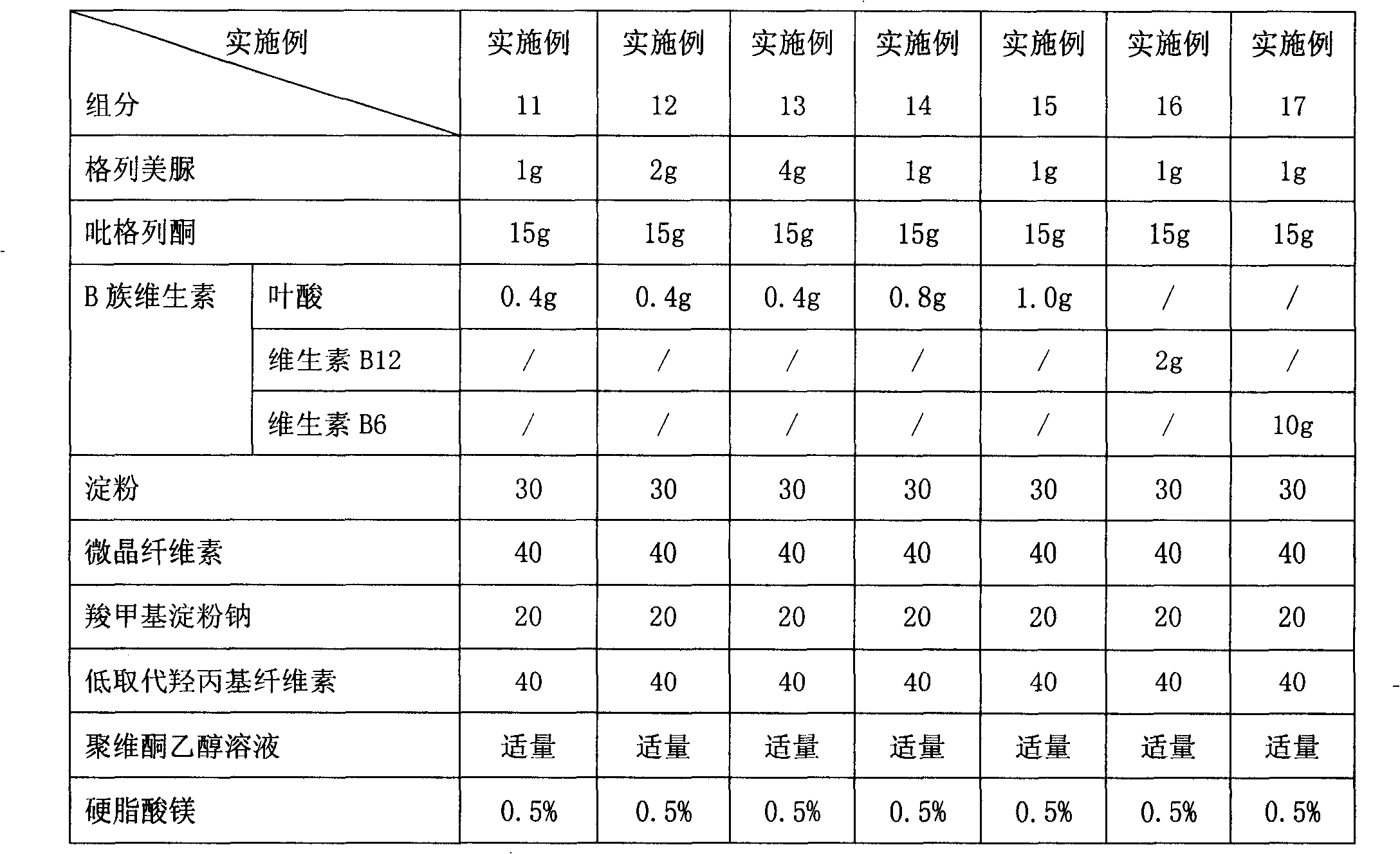
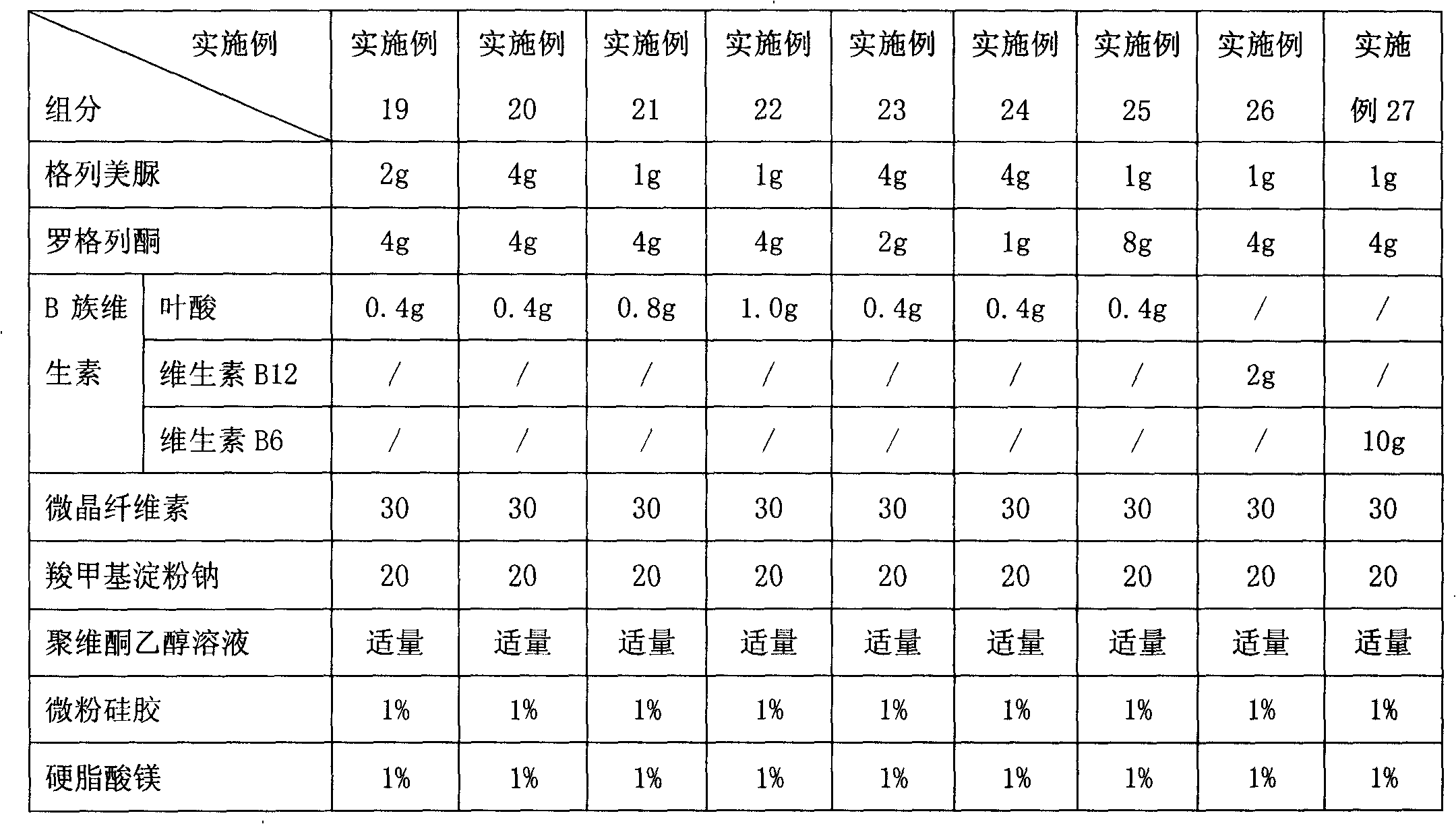
[0142] Embodiment 2 to Embodiment 8 Preparation of glimepiride rosiglitazone folic acid tablets containing different content ratios
[0143] The preparation method is the same as in Example 1, and the granules obtained according to the formula shown in Table 1 are made into tablets.
Embodiment 9
[0144] Example 9 Preparation of compound glimepiride rosiglitazone vitamin B12 (2mg) tablets (quantity of 1000 tablets)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com