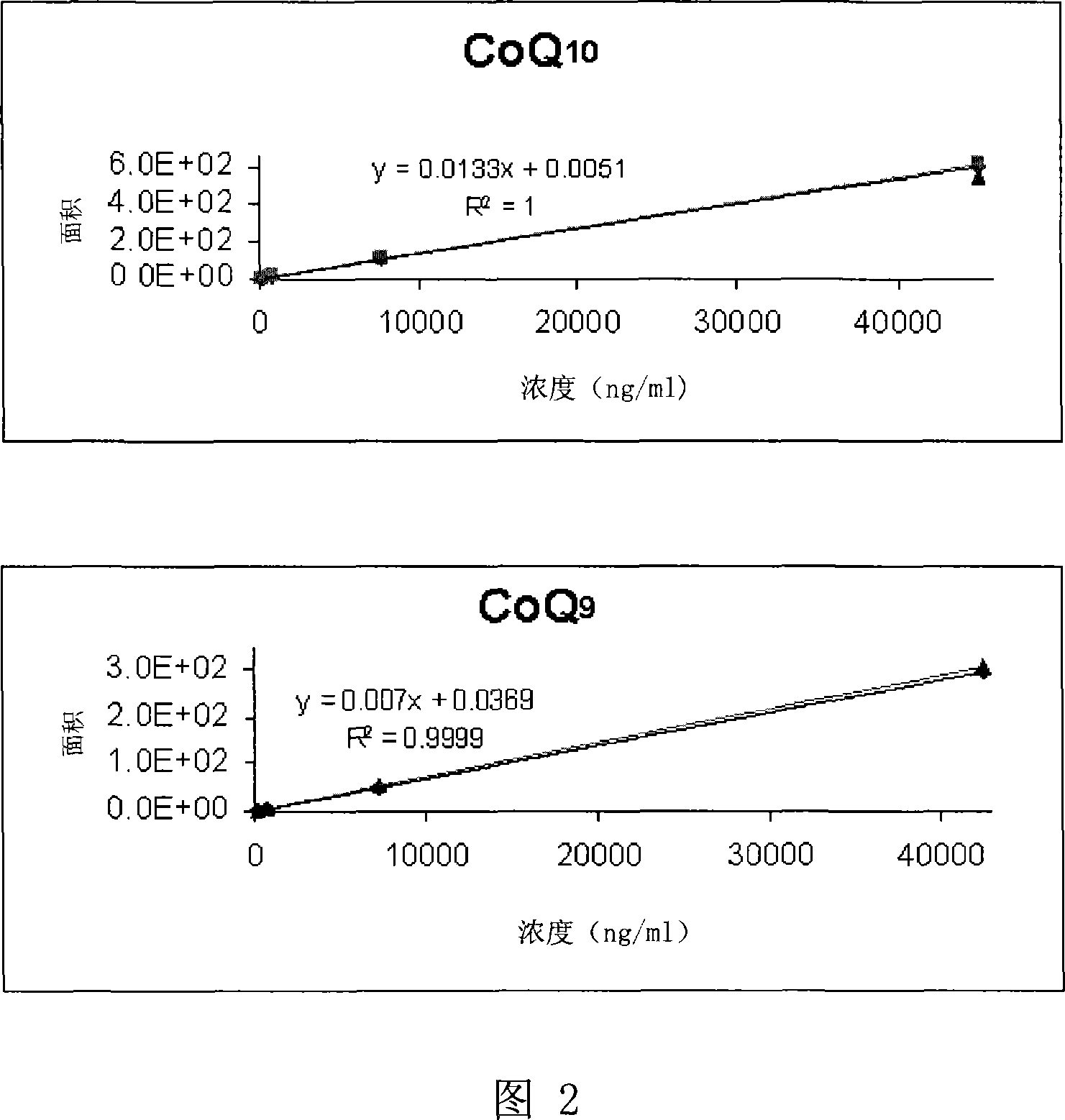Carrier used for medicine giving for intestine
A carrier and drug delivery technology, used in antipyretics, drug combinations, pharmaceutical formulations, etc., and can solve problems such as major changes in bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
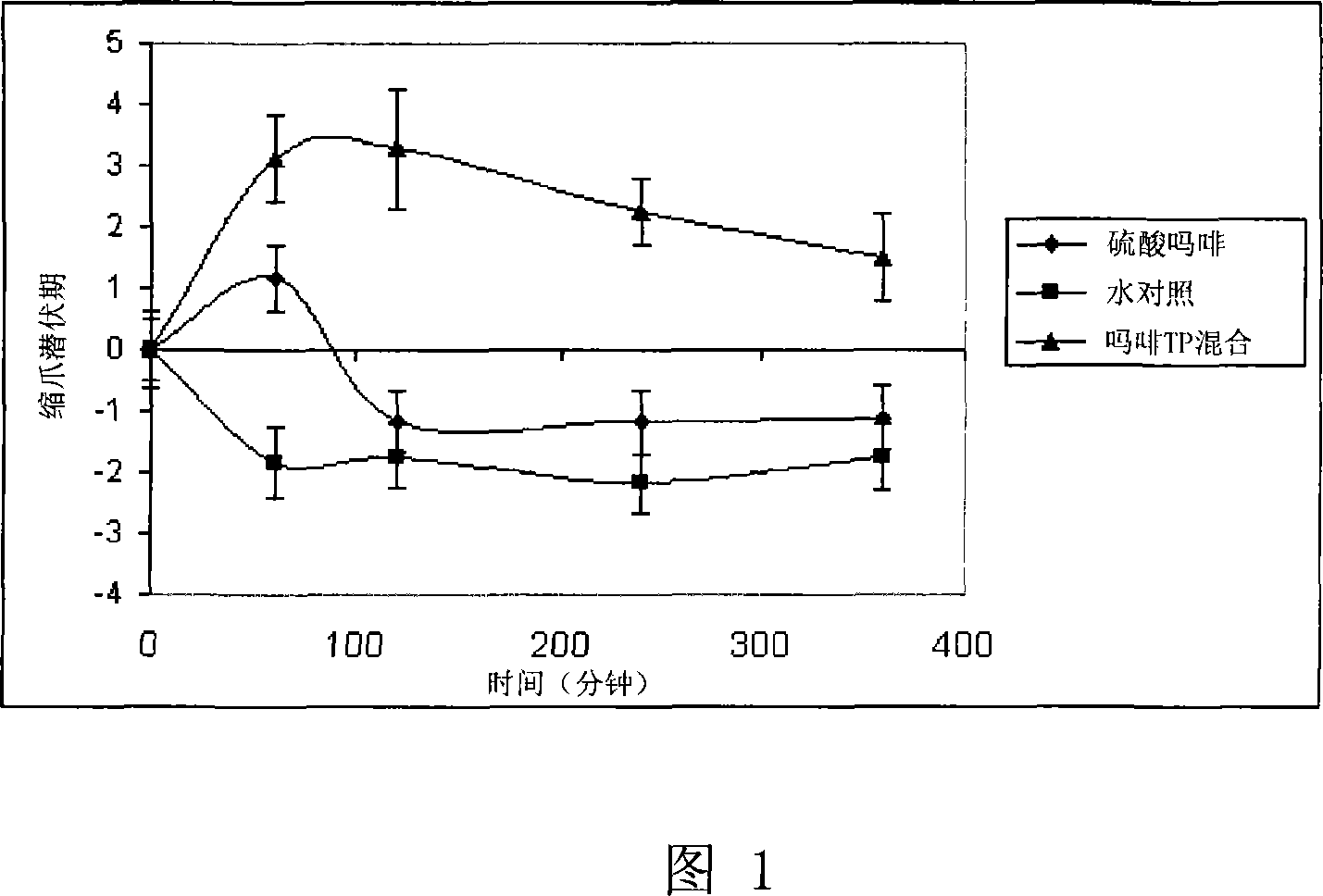
[0049] In this experiment, the efficacy was compared between the morphine composition of the present invention and morphine sulfate (currently used enteral formulation of morphine). The effect of the drug was measured by comparing the time it took for morphine-treated and non-morphine-treated rats to withdraw their paws after exposure to heat.
[0050] Material
[0051] Animals: Nine conscious Sprague-Dawley rats, each weighing 350-450 grams.
[0052] Treatment group:
[0053] 1. Control: water,
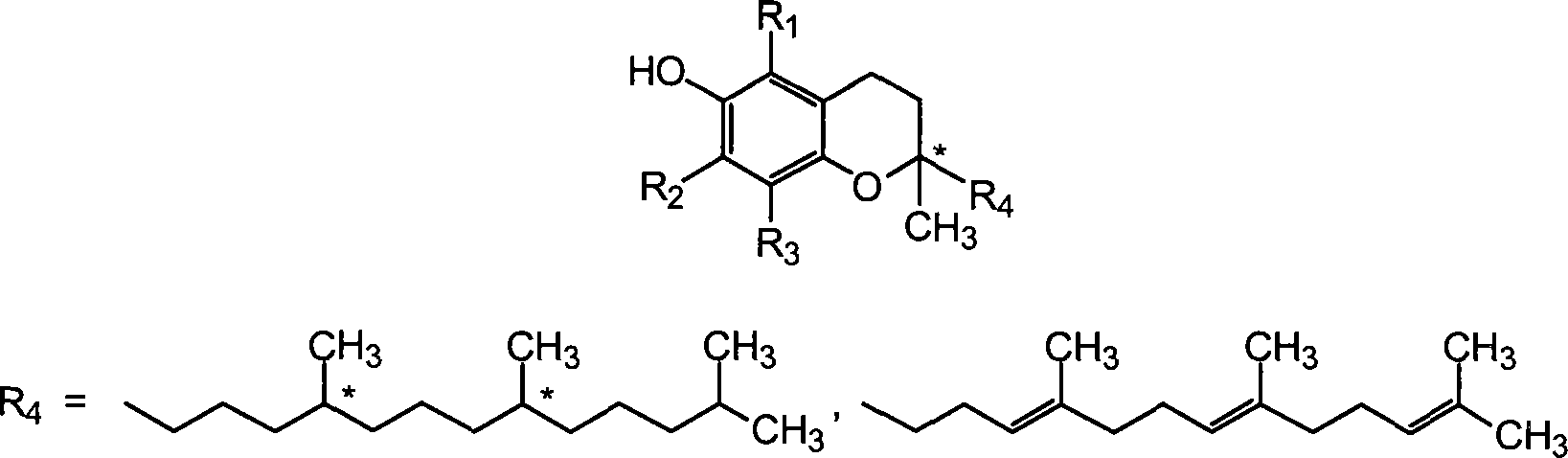
[0055] 3. Morphine with TPm: Morphine hydrochloride (14%) (TPm) in a carrier containing water (59%) and tocopheryl phosphate mixture (27%). The TPm contains monotocopheryl phosphate and ditocopheryl phosphate.
[0056] Formulations 2 and 3 were diluted with water to achieve a final morphine concentration of 5 mg / ml. For example, 0.357 grams of Formulation 3 is mixed with 0.643 grams of water to give a final concentration of morphine of 5%. The liq...
example 2
[0070] This example studies the bioavailability of coenzyme Q10 (CoQ10) in guinea pigs after administration of the following formulations:
[0071] A. CoQsol
[0072] B. CoQsol and TPM in MCT
[0073] C.MCT oil (control)
[0074] Materials and methods
[0075] preparation
[0076] Tocopheryl phosphate mixture (TPM) containing 2:1 w / w mono-tocopheryl phosphate (TP) and di-tocopheryl phosphate (T2P) was manufactured by Phosphagenics.
[0077] CoQsol was purchased from American Doctor's Trust Vitamins company.
[0078] Medium-chain triglycerides (MCT) were manufactured by Abitec Corporation of the United States.
[0079] These preparations include:
[0080] A. CoQsol: Each softgel contains 60mg CoQ and 0.44ml oily content of the pill. So the concentration of CoQ in the capsule contents is 60mg / 0.44ml=136mgCoQ / ml. CoQsol formulations also contain 136IU d-alpha-tocopherol and 3705IU vitamin A per milliliter. Excipients are rice bran oil, gelatin, glycerin, water, beeswax, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com