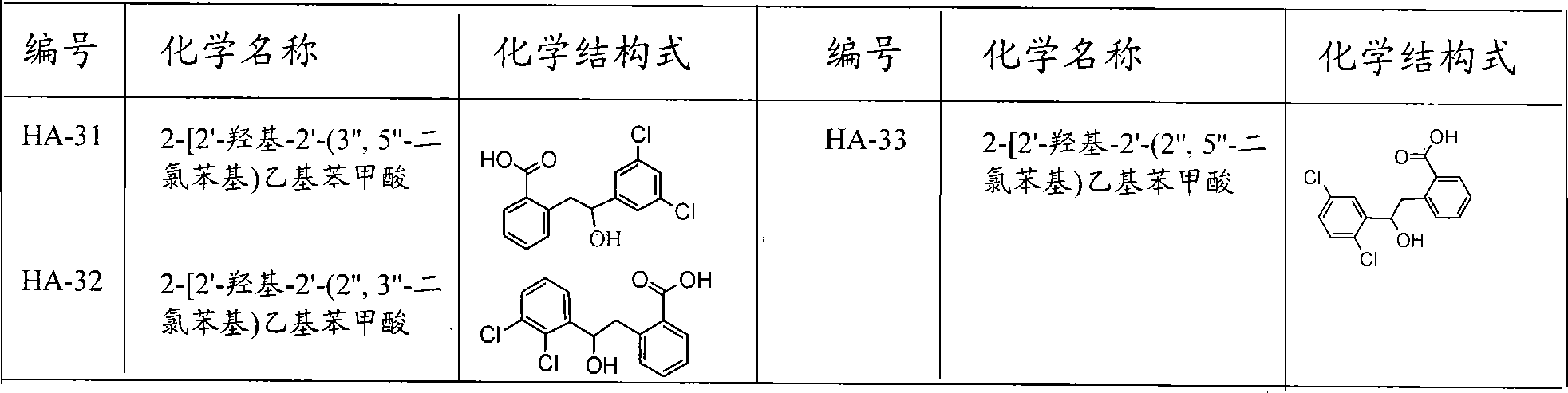
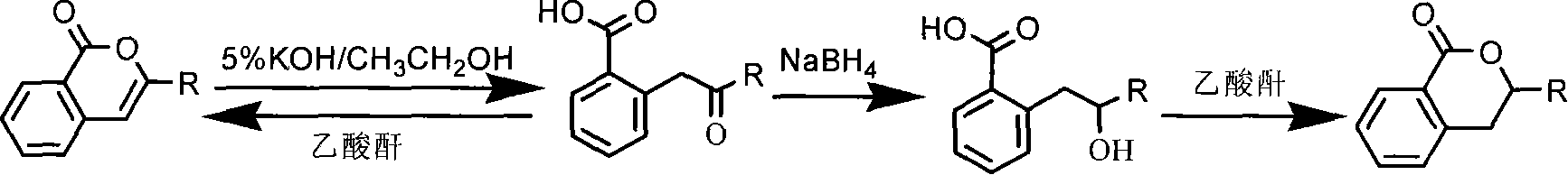Preparation and usage of unnatural 3,4-dihydro isocoumarin derivatives
A technology of isocoumarins and derivatives, applied in 3 fields, can solve problems such as relatively few reports of biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
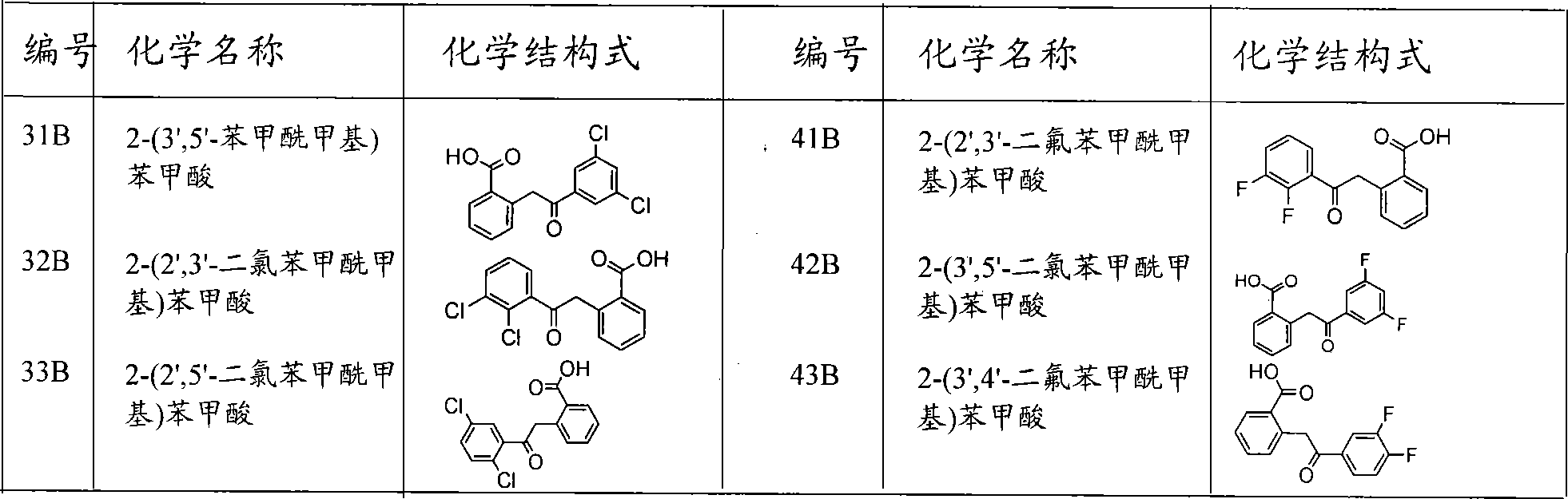
[0033] (31B) Synthesis and structure identification of 2-(3′,5′-phenacylmethyl)benzoic acid:
[0034] Dissolve 3-(3 ', 5'-dichlorophenyl) isocoumarin ( 9.0 g, 30 mmol) (31A), refluxed for 4 hours, after cooling, ethanol was removed under reduced pressure. Add cold water (20 ml) to the reaction mixture, acidify with dilute hydrochloric acid, extract 3 times with 20 ml of dichloromethane, dry over anhydrous sodium sulfate, distill off the solvent under reduced pressure to obtain a crude product, recrystallize with methanol to obtain 2-(3 ', 5'-dichlorophenacyl) benzoic acid (31B) pure product 6.5g, 21.1 mmoles, measure melting point and 1 H NMR, its chemical structural formula and physical and chemical parameters are shown in Table 1 and Table 2, as seen from Table 1 and Table 2, this compound 1 H NMR shows the chemical shift corresponding to its structure, the number of H is consistent with its structure, and MS measurement shows the corresponding M + The m / z peak of the IR ...
Embodiment 2
[0036] Synthesis and structure identification of 2-(2′,3′-dichlorophenacyl)benzoic acid (32B):
[0037] Dissolve 3-(2 ', 3'-dichlorophenyl) isocoumarin (8.8 g, 30 mmol) in a 100 ml three-necked flask with ethanol (50 ml) and potassium hydroxide (5%, 100 ml) (32A), refluxed for 4 hours, after cooling, ethanol was removed under reduced pressure. Add cold water (20 ml) to the reaction mixture, acidify with dilute hydrochloric acid, extract 3 times with 20 ml of dichloromethane, dry over anhydrous sodium sulfate, distill off the solvent under reduced pressure to obtain a crude product, recrystallize with methanol to obtain 2-(2 ', 3'-dichlorophenacyl) benzoic acid (32B) pure product 6.8g, 22.0 mmoles, measure melting point and 1 H NMR, its chemical structural formula and physical and chemical parameters are shown in Table 1 and Table 2, as seen from Table 1 and Table 2, this compound 1 H NMR shows the chemical shift corresponding to its structure, the number of H is consistent w...
Embodiment 3
[0039] Synthesis and structure identification of 2-(2′,5′-dichlorophenacyl)benzoic acid (33B):
[0040] Dissolve 3-(2′,3′-dichlorophenyl)isocoumarin (9.0g, 30mmol) in a 100ml three-necked flask with ethanol (50ml) and potassium hydroxide (5%, 100ml) (32A), refluxed for 4 hours, after cooling, ethanol was removed under reduced pressure. Add cold water (20 ml) to the reaction mixture, acidify with dilute hydrochloric acid, extract 3 times with 20 ml of dichloromethane, dry over anhydrous sodium sulfate, distill off the solvent under reduced pressure to obtain a crude product, recrystallize with methanol to obtain 2-(2 ', 5'-dichlorophenacyl) benzoic acid (33B) pure product 6.3g, 20.5 mmoles, measure melting point and 1 H NMR, its chemical structural formula and physical and chemical parameters are shown in Table 1 and Table 2, as seen from Table 1 and Table 2, this compound 1 H NMR shows the chemical shift corresponding to its structure, the number of H is consistent with its ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com