Organism indwelling support
A biological and biodegradable technology, applied in the field of biological indwelling stents, can solve the problems of low degree of sustained release, inability to obtain sustained release of restenosis rate, etc., and achieve the effect of easy provision.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
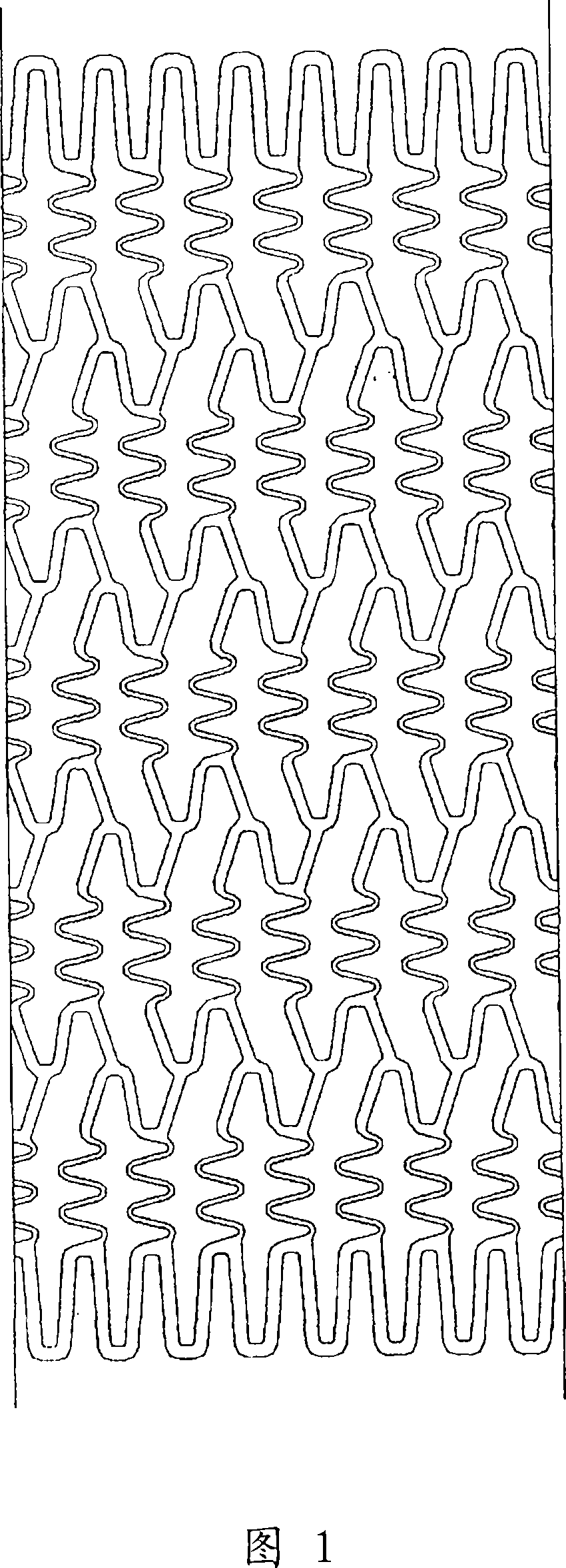
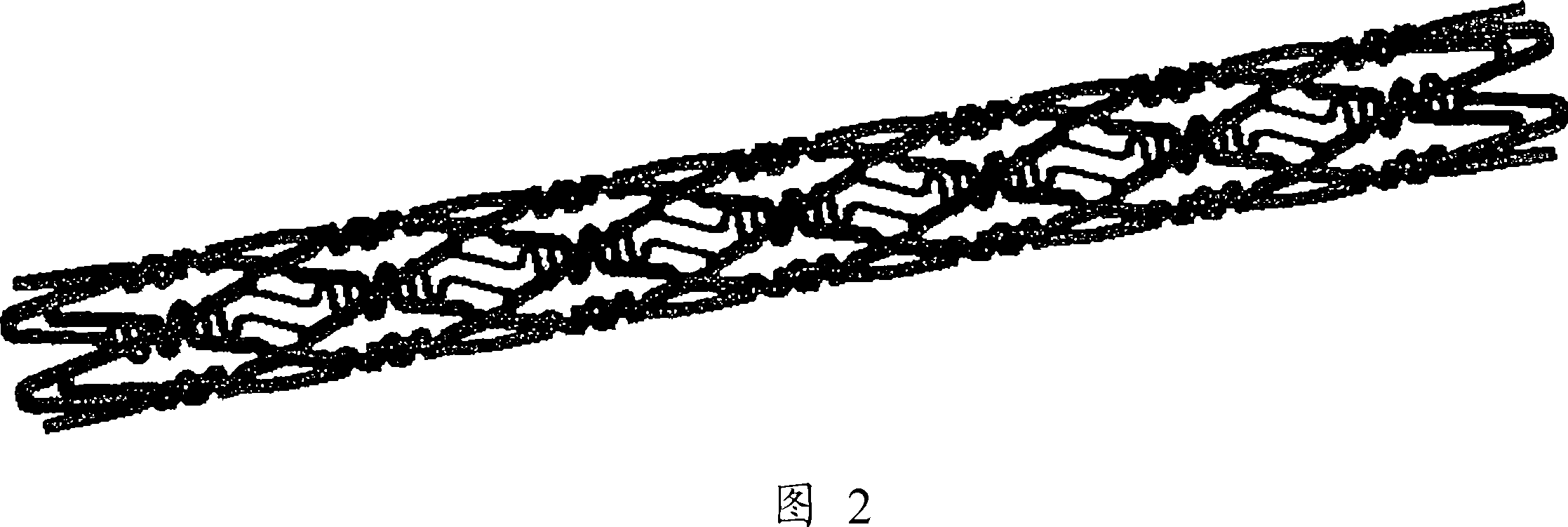
[0062] The main body of the stent was produced by laser cutting a cylindrical tube of stainless steel (SUS316L) with an inner diameter of 1.50 mm and an outer diameter of 1.80 mm into a stent pattern, and electrolytically polishing it. The expanded view of the used bracket is shown in FIG. 1 , and the schematic diagram is shown in FIG. 2 . The length of the stent was 13 mm, the thickness was 120 μm, and the nominal diameter after expansion was 3.5 mm. Since the stent is a so-called balloon-expandable type, it is a type of stent that is expanded and indwelling by using a balloon catheter having a balloon near the distal end of the catheter. Balloon-expandable stents are installed in a state in which the balloon of the balloon catheter is partially deflated, and after being delivered to the target site, the stent is expanded and indwelling by expanding the balloon.
[0063] Lactic acid-glycolic acid copolymer (product number: 85DG065, Absorbale Polymers International company, l...
Embodiment 2
[0067] In addition to making a solution of drug concentration / polymer concentration=0.75wt% / 0.50wt%, forming an inner layer with a polymer weight of 66 μg and a drug weight of 100 μg per stent (drug / polymer weight ratio=1.52 ) except that it was produced in the same manner as in Example 1.
[0068] The total polymer weight of the inner and outer layers of each obtained stent was 258 μg, and the drug weight was 150 μg (drug / polymer weight ratio=0.58).
Embodiment 3
[0070] In addition to making a solution of drug concentration / polymer concentration=0.05wt% / 0.50wt%, forming an outer layer with a polymer weight of 500 μg and a drug weight of 50 μg per stent (drug / polymer weight ratio=0.10 ) except that it was produced in the same manner as in Example 1.
[0071] The total polymer weight of the inner and outer layers of each obtained stent was 600 μg, and the drug weight was 150 μg (drug / polymer weight ratio=0.25).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com