Matrine eye drip liquid, and producing method
A technology of matrine and eye drops, which is applied in the field of human medicine and can solve the problems of large volume, inconvenience for patients, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
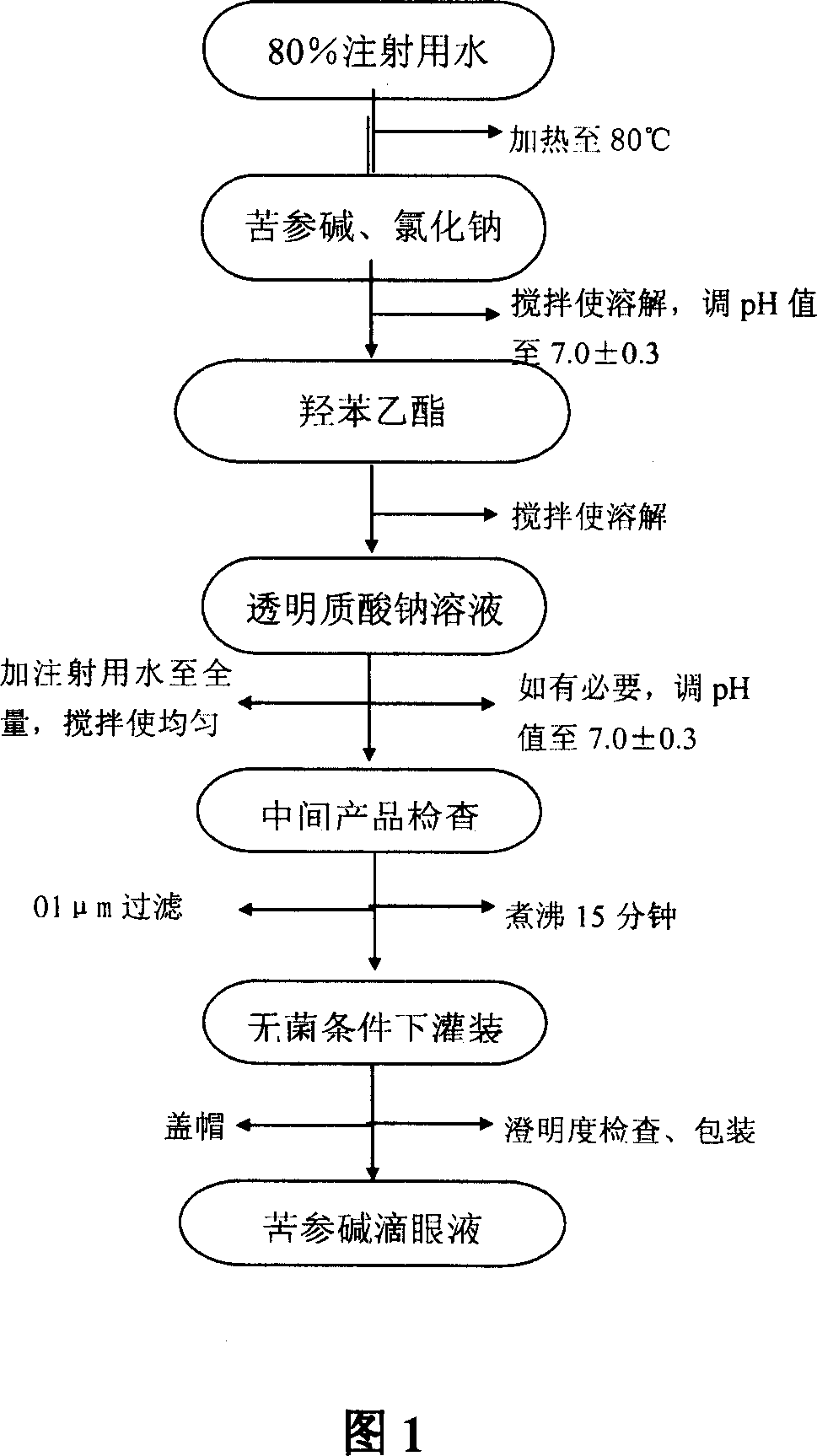
[0047] Example 1: Operation method
[0048] (1) Add 80% water for injection in the liquid preparation container and heat to 80°C;
[0049] (2) Add matrine and sodium chloride, stir to dissolve; adjust the pH value to 7.0±0.3
[0050] (3) Add ethyl p-hydroxybenzoate, stir to dissolve;
[0051] (4) Add sodium hyaluronate solution (swell and dissolve with a small amount of water for injection in advance);
[0052] (5) Add water for injection to the full amount, and stir evenly;
[0053] (6) The pH value of the solution should be 6.7-7.3, if necessary, adjust with dilute hydrochloric acid or sodium hydroxide;
[0054] (7) After passing the inspection of the intermediate product, boil it for 15 minutes;
[0055] (8) Filtration with a 0.1 μm sterile filter;
[0056] (9) Fill it in an 8ml plastic eye drop bottle under aseptic conditions, and cover the anti-theft cap.
[0057] (10) Check the clarity and pack it.
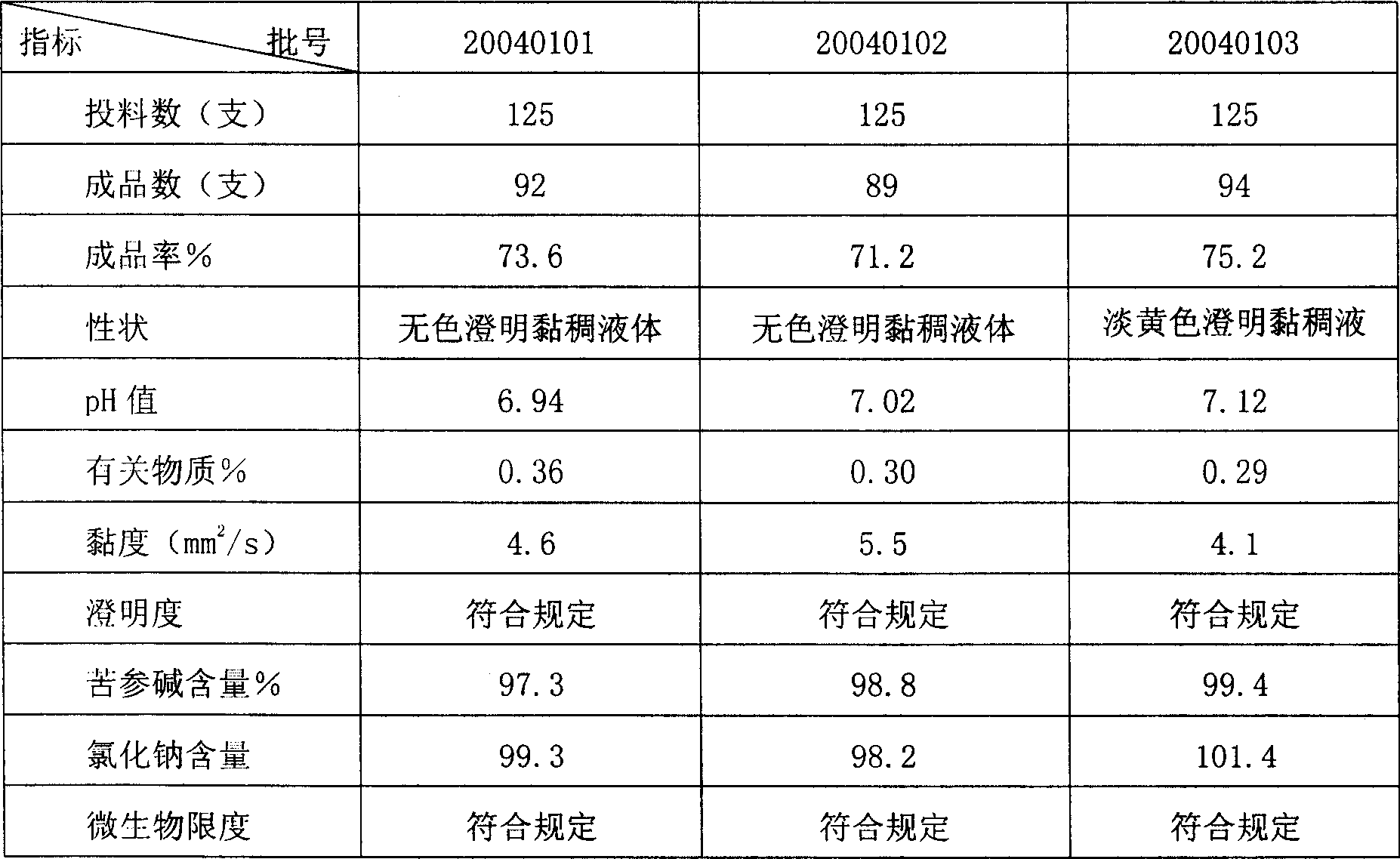
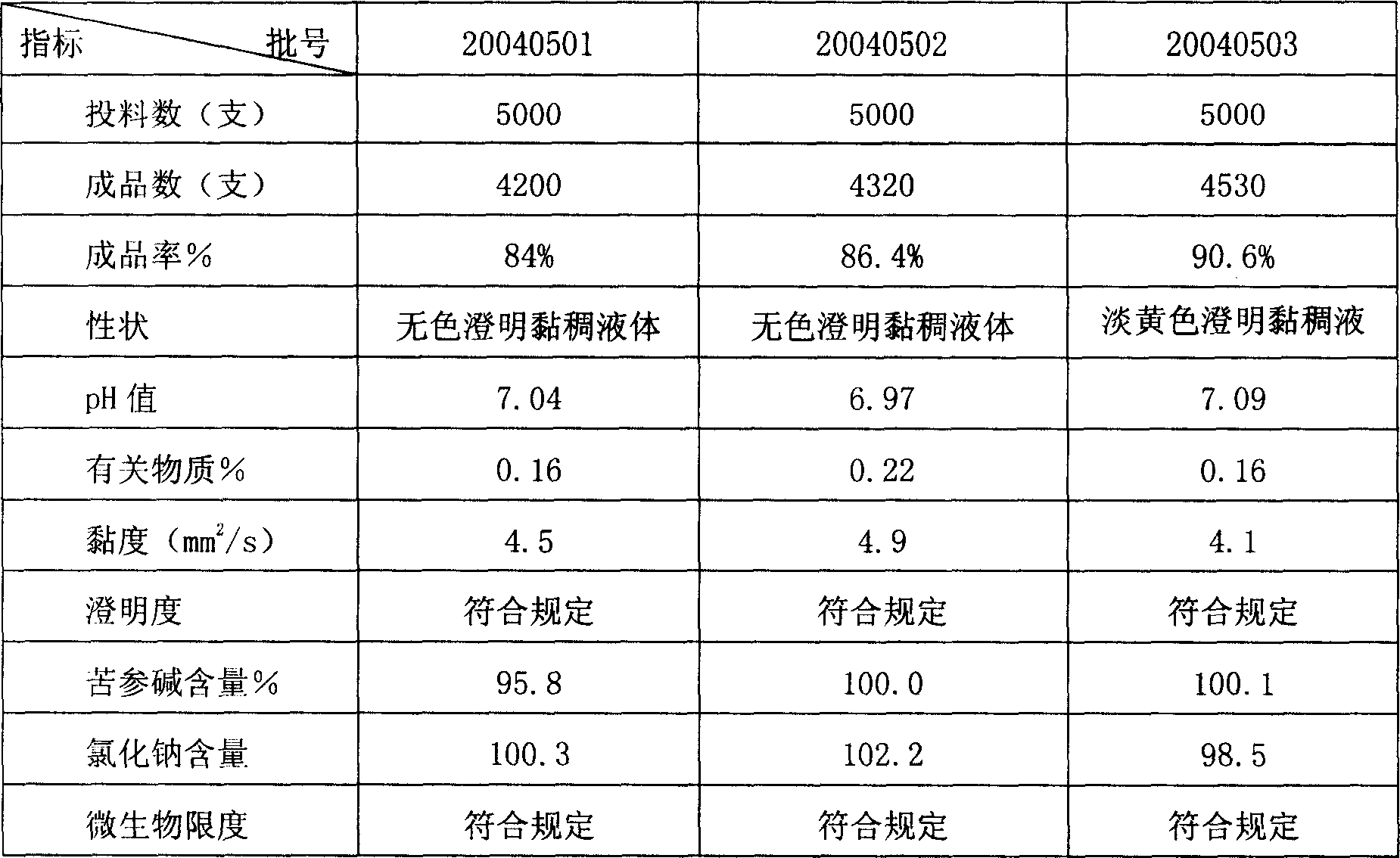
[0058] The above ingredients and weight percentage are: 0.25% of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com