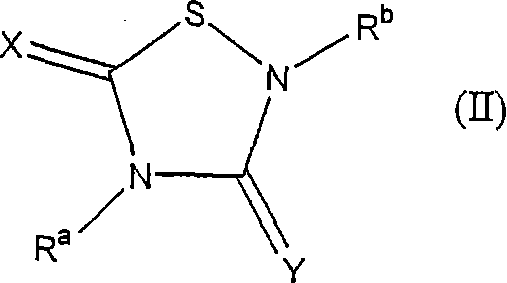
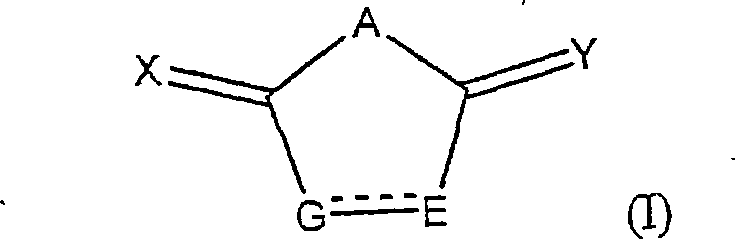
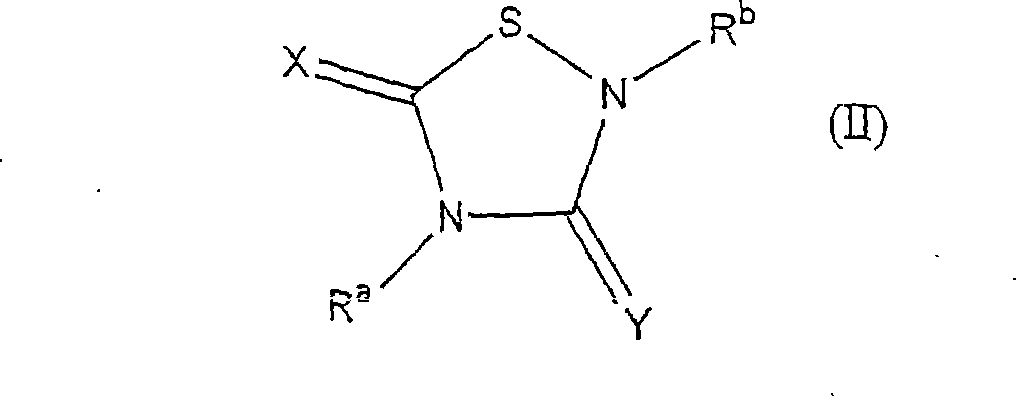
Heterocycle inhibitor for glycogen synthase kinase gsk-3
A compound and general formula technology, applied in the field of glycogen synthase kinase 3β, can solve the problems of activity upregulation and unclear exact function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1 - Enzyme Inhibition of Compositions of the Invention
[0084] GSK-3β inhibition: in the presence or absence of the corresponding test compound, by incubating a mixture of GSK-3 enzyme (Sigma), phosphate (ester) source and GSK-3 substrate, and by measuring the GSK of the mixture -3 activity to determine GSK-3 activity.
[0085] Specifically, in supplementation with 15 µM (final concentration) of synthetic peptide GS 1 as a substrate [Woodgett, J.R. "Use of peptides for affinity purification of protein-serine kinases", Anal. Biochem., 1989, 180, 237-241], 15 μM ATP, 0.2 μC j [ -32 P]ATP and different concentrations of test compounds in a final volume of 12 μl buffer (50 mM tris, pH=7.5, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 10 mM Cl 2 Mg), GSK-3 activity was determined by incubating the enzyme at 37°C for 20 minutes. Reactions were terminated by adding an aliquot of the reaction mixture to phosphocellulose p81 paper. The papers were washed three times with 1% pho...
Embodiment 2
[0101] Example 2 - Analysis of neurite outgrowth after drug treatment
[0102] Cells were maintained in Dulbecco's medium (DEMEM) containing 10% fetal bovine serum, glutamine (2 mM) and antibiotics. To analyze potential GSK-3 inhibitory effects in vivo, mouse neuroblastoma (neuroblastoms) N 2 A culture (Garcia-Perez, J.; Avila, J.; Diaz-Nido, J. "Lithium induces morphological differentiation of mouse neuroblastoma", J. Neurol. Res., 1999, 57, 261-270). Test compounds are added to these cell cultures. This cell line was characterized to express a certain neuronal phenotype (neuritic elongation) after addition of lithium chloride (10 mM), a known GSK-3 inhibitor. After 2-3 days of incubation, the effects of those tested compounds collected in Table 1 were examined. It was found that axonal elongation was produced more than when lithium was added. This fact confirms the inhibitory effect of the compounds of the present invention on GSK-3 in vivo.
Embodiment 3
[0103] Example 3. Cell cycle arrest
[0104] in parallel, at N 2 Potential disruption of the cell cycle by these compounds was studied in A cells. The cell culture was maintained in Dulbecco's medium (DEMEM) containing 10% fetal bovine serum, glutamine (2 mM) and antibiotics.
[0105] The first 4 compounds of general formula (I) collected in Table 3 were analyzed under the conditions described and showed the ability to inhibit the cell cycle at inhibitor concentrations of 100 nM and 1 μM. Cellular blockade was initially observed at concentrations of 100-200 nM and was fully effective at a concentration of 1 μM.
[0106] The compounds tested were not toxic in the resting fibroblast culture MRC-5 after 10 days of continuous exposure to the inhibitor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com