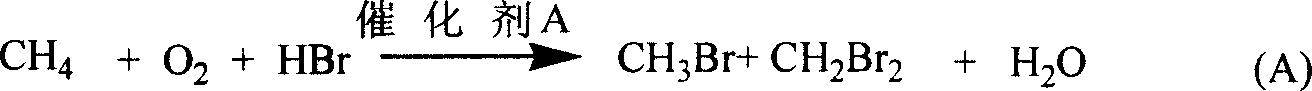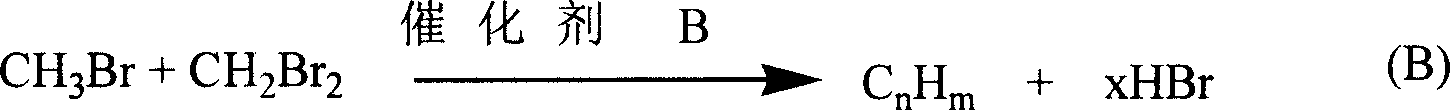Flow process for synthesizing C3 to C13 high hydrocarbons by methane through non-synthetic gas method
A technology of methane and process, which is applied in the field of preparation of high-carbon hydrocarbons from C3 to C13, and can solve problems such as the danger of large-scale use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1 to example 23
[0012] Example 1 to Example 23, the bromine oxidation reaction of alkanes
[0013] The catalyst is made of silica (10 g specific surface 1.70m 2 / g), RuCl 3 The solution (0.00080 g Ru / ml) and the corresponding metal nitrate solution (0.10 M) were mixed according to the mol composition of the catalyst in Table 1, stirred at room temperature for half an hour, dried at 110°C for 4 hours, and finally fired at 450°C Catalyst Examples 1 to 23 in Table 1 were obtained after 12 hours.
[0014] The catalytic reaction is carried out in a quartz tube reactor with an internal diameter of 0.80 cm and a length of 60 cm. The reaction temperature is listed in Table 1. The methane flow is 5.0 ml / min, the oxygen flow is 5.0 ml / min, and 40 wt% HBr / H 2 O aqueous solution flow rate is 4.0 milliliters (liquid) / hour, catalyst 1.0000 grams. Both ends of the catalyst are filled with quartz sand. The reaction products were analyzed by gas chromatography, and the results are listed in Table 1, as in...
example 24
[0018] The catalyst is made of silica (10 g specific surface 0.50m 2 / g), RuCl 3 solution (0.0008 g Ru / ml), La(NO 3 ) 3 (0.10M), Ba(NO 3 ) 2 (0.10M), Ni(NO 3 ) 2 (0.10M) by 2.5% La, 2.5% Ba, 0.5% Ni, 0.1% Ru and 94.4% SiO 2 Mix the mol composition, stir at room temperature for half an hour, dry at 110°C for 4 hours, and finally burn at 450°C for 12 hours to get the composition La2.5%Ba2.5%Ni0.5%Ru0.1% / SiO 2 catalyst.
[0019] The catalytic reaction is carried out in a quartz tube reactor with an inner diameter of 1.50 cm and a length of 60 cm. The reaction temperature is 660° C., the methane flow rate is 15.0 ml / min, the oxygen flow rate is 5.0 ml / min, and 40 wt% HBr / H 2 O aqueous solution flow rate is 6.0 milliliters (liquid) / hour, catalyst 5.000 grams. Both ends of the catalyst are filled with quartz sand. The reaction product is analyzed on gas chromatography, and the result is: methane conversion rate 32.0%, CH 3 The selectivity of Br is 80.8%, CH 2 Br 2 The s...
example 25 to example 38
[0020] Example 25 to Example 38, brominated hydrocarbons are converted into higher carbon hydrocarbons
[0021] Preparation of ZnO / HZSM-5 and MgO / HZSM-5 Catalysts
[0022] Catalysts C1 to C14 of examples 25 to 38 in table 2 are made of molecular sieve HZSM-5 (Si / Al=360,283m 2 / g), water and Zn(NO 3 ) 2 ·6H 2 O(or Mg(NO 3 ) 2 ·6H 2 O) Mix and stir according to the amount in Table 2, soak at room temperature for 12 hours, dry at 120°C for 4 hours, burn at 450°C for 8 hours, press at 100 atmospheres, crush and sieve to 40 to 60 mesh to obtain Catalysts in Table 2.
[0023] example
[0024] The catalysts of Examples 25 to 38 were used for CH 3 Br is converted into the reaction of high-carbon hydrocarbons, the reaction is carried out in a glass reaction tube with an inner diameter of 1.5 cm, the catalyst consumption is 8.0 grams, the reaction temperature is 240 ° C, CH 3 Br flow rate is 6.8ml / min, reaction product is analyzed on gas chromatography, CH 3 The con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com