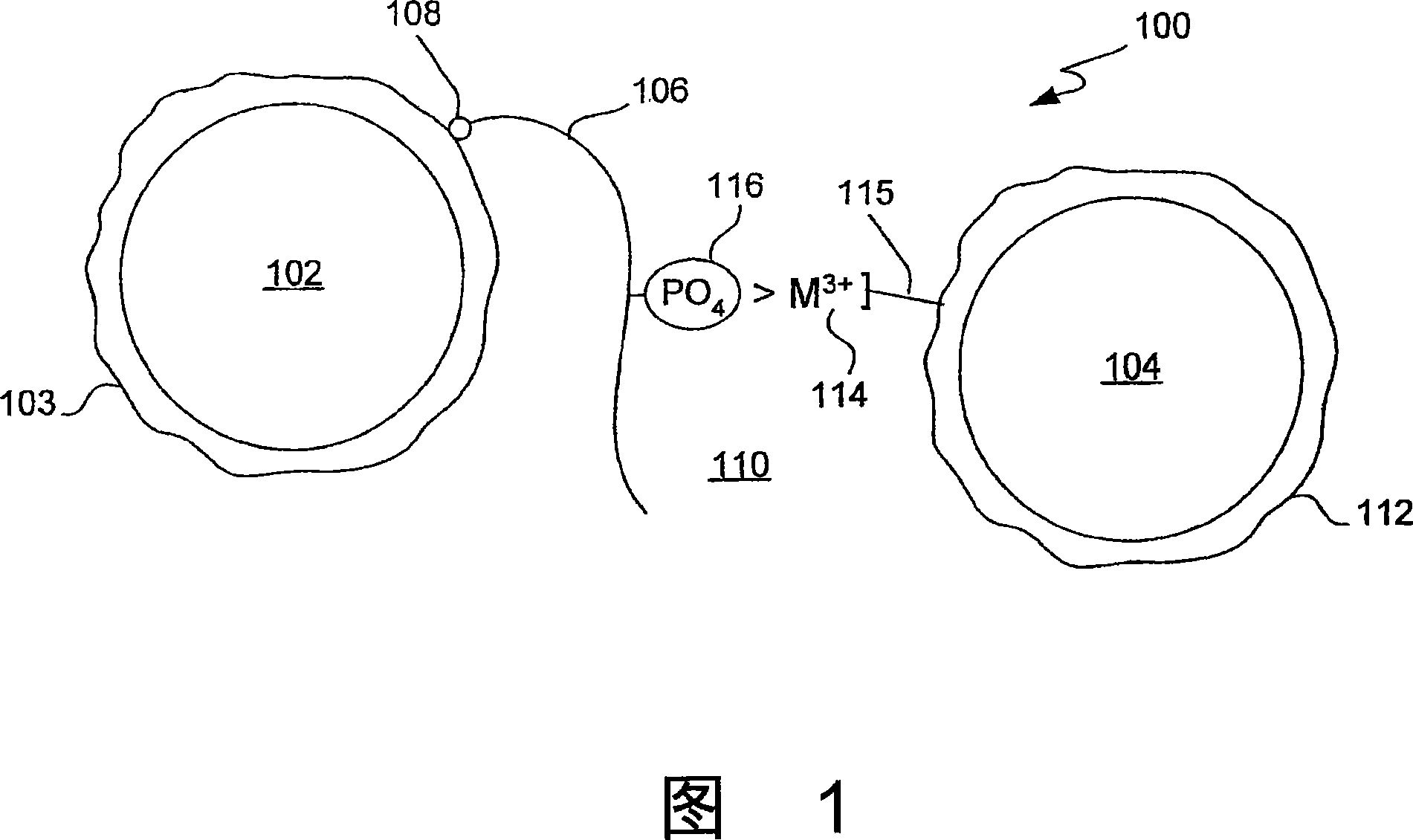
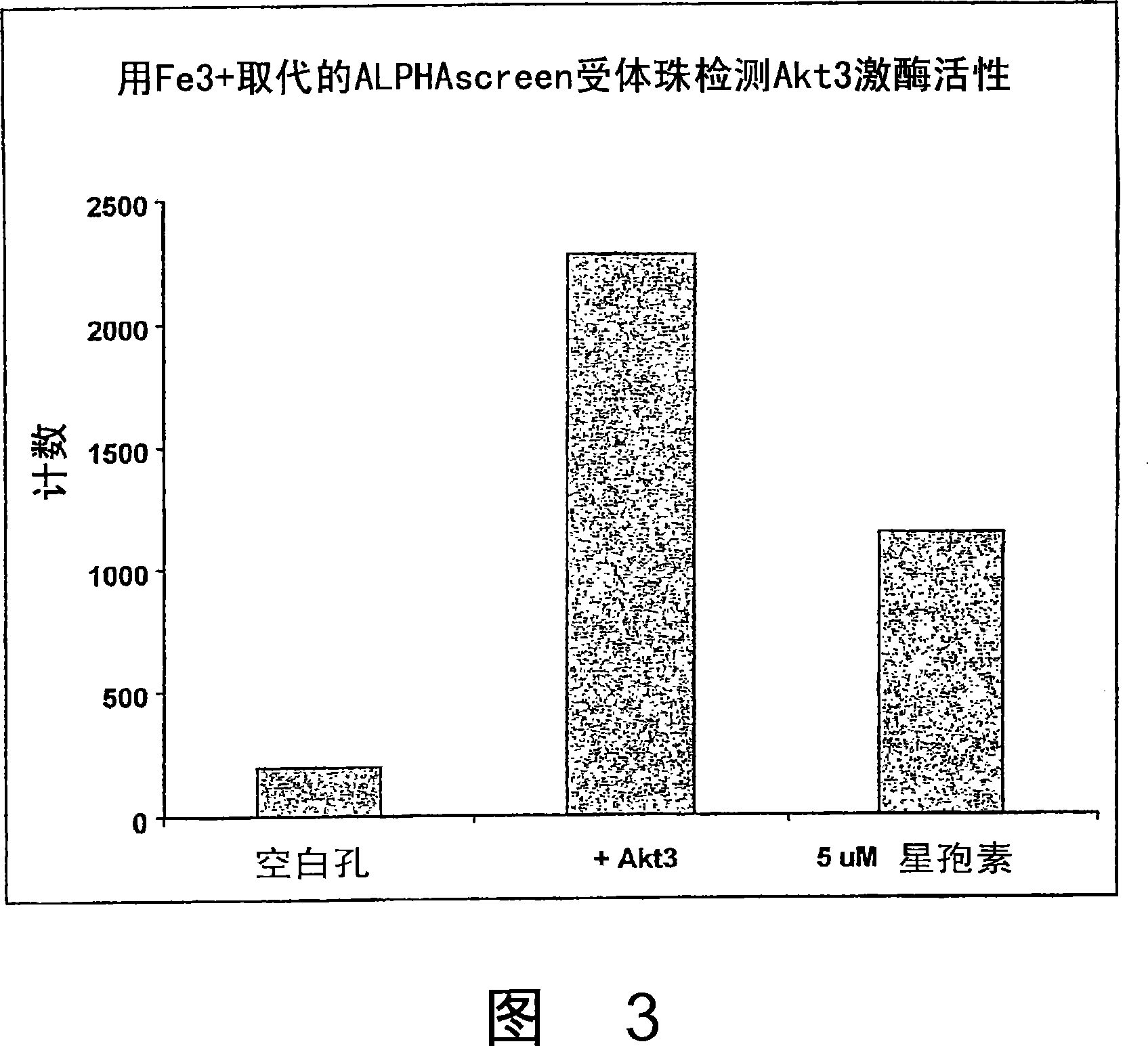
Trivalent metal mediated homogeneous luminescent proximity assay
A trivalent metal, chemiluminescent technology, applied in the field of biological experiments, biological experiments, can solve the problems affecting the total effectiveness and repeatability of the HTS program, can not be used, high inherent polarization and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0044] The present invention can be embodied as composition, kit or method, for example:
[0045] A composition comprising donor particles comprising a photosensitizer; acceptor particles comprising a chemiluminescent compound characterized in that it produces a luminescent signal when the photosensitizer is activated and the donor and acceptor particles are in proximity; and a complex Trivalent metal ions bound to the surface of one of the donor or acceptor particles.
[0046] A composition comprising polymer particles comprising trivalent metal ions complexed to the surface of the particles and comprising one of a photosensitizer and a chemiluminescent compound. The particle may be characterized as producing a luminescent signal when a second particle containing the other of the photosensitizer and the chemiluminescent compound is in proximity and the photosensitizer is activated.
[0047] A kit for an assay comprising, in packaging combination: a reagent for performing an ...
Embodiment 1
[0054] Materials and methods
[0055] AlphaScreen TM Streptavidin Donor Beads, AlphaScreen TM Ni 2+ - Chelation acceptor beads and AlphaScreen TM Unconjugated "raw" acceptor beads were purchased from Perkin Elmer Life and Analytical Sciences (Boston, MA). IMAP TM Binding buffer was obtained from Molecular Devices (Sunnyvale, CA) as a 5x concentrate. Trivalent metal ion salts were purchased from Sigma Aldrich, St. Louis, MO (FeCl 3 ) and Alfa Aesar, Ward Hill, MA (GaCl 3 and AlCl 3 ). Akt3 kinase and biotinylated Crosstide substrate were purchased from Upstate, Charlottesville, VA. Sodium cyanoborohydride and carboxymethyl-L-lysine were purchased from SigmaAldrich. Other solutions described herein were prepared from research grade materials obtained from VWR International, West Chester, PA. Initially, the complexation of trivalent metal ions to AlphaScreen was developed TM Two methods of acceptor beads are described below.
[0056] Method 1: Replace Ni with trivalen...
Embodiment 2
[0086] Example 2: Direct coupling of trivalent metal ions to AlphaScreen via 1-propylamino-4-acetoxy-1,4,7-triazacyclononane TM acceptor beads
[0087] Trivalent metal ions can be coupled to AlphaScreen via 1-propylamino-4-acetoxy-1,4,7-triazacyclononane or another N-containing heterocycle TM Acceptor beads, coupled in the same manner as described above in Method 2 via NTA coupling. Briefly, the original AlphaScreen TM Aldehyde acceptor beads can be mixed with 1-propylamino-4-acetoxy-1,4,7-triazacyclononane, Tween-20 and NaCNBH4 (sodium cyanoborohydride) buffer solution. The reaction was incubated with shaking at 37°C in the dark for 48-60 hours, then 1M Tris-HCl, pH 7.5 was added to block the reaction, and then incubated at 37°C in the dark for 1 hour. The beads were then centrifuged, the supernatant decanted and the pelleted beads resuspended in 0.1M Tris-HCl, pH8. Repeat this centrifugation and resuspension process twice, then resuspend the beads in 100 mM M prepared in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com