Triazole compounds and their use as metabotropic glutamate receptor antagonists
A compound, halogenated alkyl technology, applied in the field of new compounds, which can solve problems such as increased release of neurotransmitters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
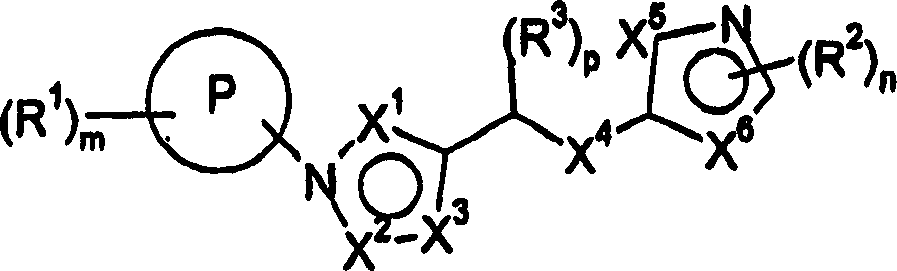
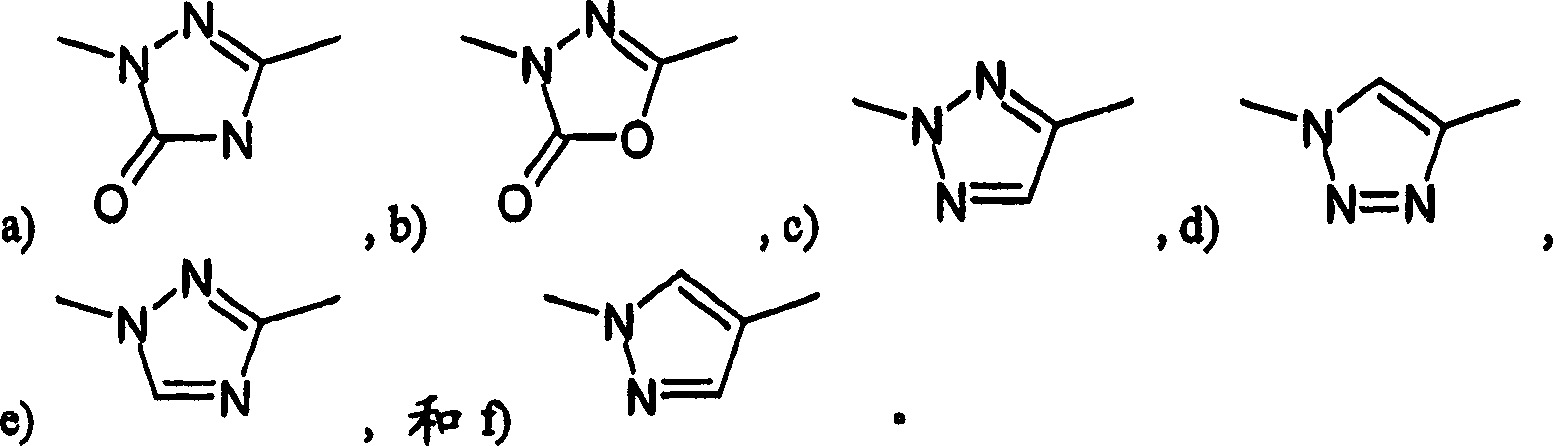
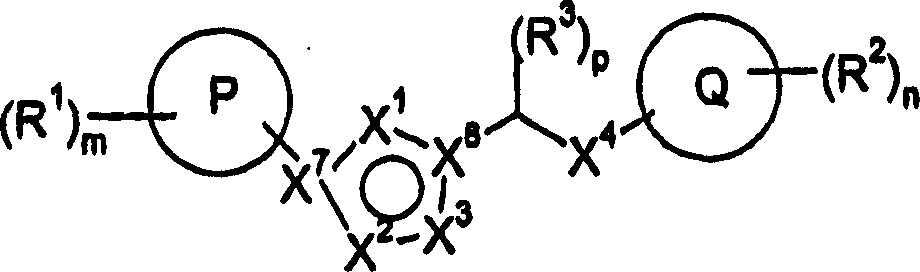
[0271] The present invention also relates to the following compounds which can be used as intermediates in the preparation of compounds of formula I:
[0272] Methyl-(4-methyl-5-pyridin-4-yl-4H-[1,2,4]triazol-3-yl)-amine
[0273] 4-Methyl-5-pyridin-3-yl-2,4-dihydro-3H-1,2,4-triazole-3-thione
[0274] 4-Methyl-5-pyridin-4-yl-2,4-dihydro-[1,2,4]triazole-3-thione
[0275] 4-cyclopropyl-5-pyridin-4-yl-2,4-dihydro-3H-1,2,4-triazole-3-thione
[0276] 4-(4-Methyl-5-methylsulfanyl-4H-[1,2,4]triazol-3-yl)-pyridine
[0277] 4-(4-Cyclopropyl-5-methylthio-4H-[1,2,4]triazol-3-yl)-pyridine
[0278] 4-(5-Methanesulfonyl-4-methyl-4H-[1,2,4]triazol-3-yl)-pyridine
[0279] 4-(4-Cyclopropyl-5-methylsulfonyl-4H-[1,2,4]triazol-3-yl)-pyridine
[0280] Methyl [(4-methyl-5-pyridin-3-yl-4H-1,2,4-triazol-3-yl)thio]acetate
[0281] [(4-Methyl-5-pyridin-3-yl-4H-1,2,4-triazol-3-yl)thio]acetic acid
[0282] N'-(3-chlorophenyl)-2-[(4-methyl-5-pyridin-3-yl-4H-1,2,4-triazol-3-yl)sulfanyl]acetohydrazide...
Embodiment 1
[0308] Methyl-(4-methyl-5-pyridin-4-yl-4H-[1,2,4]triazol-3-yl)-amine
[0309] 1000mg (4.35mmol) of N-amino-N', N"-dimethyl-guanidine hydrochloride (Henry; Smith; J.Amer.Chem.Soc.; 73; 1951; 1858) and 774mg (4.35mmol) A mixture of isonicotinoyl chloride hydrochloride in 3 ml of pyridine was heated at 160 °C for 5 minutes under microwave irradiation. Saturated K was added 2 CO 3 aqueous solution, the mixture was washed with CHCl 3 extraction. The combined organic layers were dried and concentrated. Recrystallization from ethanol, water and EA afforded 216 mg (26%) of the title compound. 1 H NMR (d6-DMSO): 2.85 (d, 3H) 3.45 (s, 3H) 6.25 (d, 1H) 7.65 (m, 2H) 8.67 (m, 2H)
Embodiment 2
[0311] 4-Methyl-5-pyridin-3-yl-2,4-dihydro-3H-1,2,4-triazole-3-thione
[0312] 4-methyl-3-thiosemicarbazide (902mg, 8.58mmol), nicotinic acid (960mg, 7.80), EDCl (1.64g, 8.58mmol), HOBt (1.16g, 8.58mmol) in DMF (10mL) The solution was stirred overnight at room temperature. The reaction mixture was diluted with EA (100 mL), washed successively with 10% hydrochloric acid, water, saturated Na 2 CO 3 Aqueous, water and brine washes. The organic phase was dried (Na 2 SO 4 ), filtered and concentrated in vacuo. The residue was stirred in NaOH (53.4 mL, 66.7 mmol, 5% in water) at 60 °C overnight. The mixture was cooled to room temperature and the pH was adjusted to 6 using 1N hydrochloric acid. The aqueous phase was saturated with solid NaCl and extracted with EA. The combined organic phases were washed with brine, dried (Na 2 SO 4 ), filtered, concentrated, and dried in vacuo to give the title compound (180 mg). 1 H-NMR: 11.6(br s, 1H), 8.94(s, 1H), 8.83(dd, 1H), 7.98(m, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com