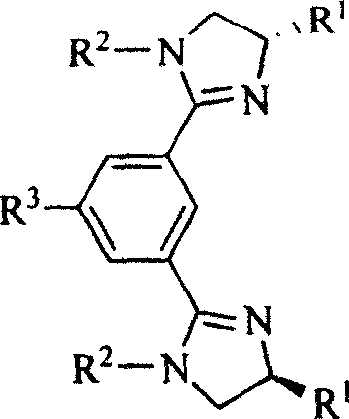
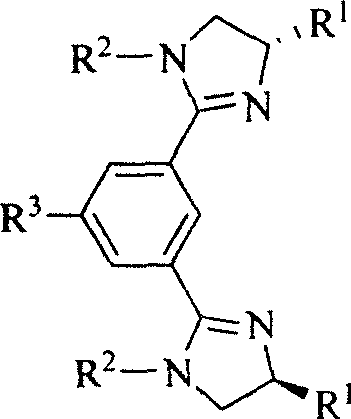
Chiral and non-chiral diimidazolinylbenzene compounds and synthesis method
A technology for bisimidazolinylbenzene and compound, which is applied in the field of chiral and achiral bisimidazolinylbenzene compounds and synthesis, can solve the problems of limited application, difficult preparation of chiral diamine and high price, and achieves good application Foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0017] Example 1, the preparation of 1,3-bis(4,5-dihydro-1-p-tolyl-1H-imidazol-2-yl)benzene: N,N'-bis(2-hydroxyethyl)-1, A solution of 3-dibenzamide (2.4 mmol) in thionyl chloride (3 mL, 41.3 mmol) was stirred under reflux for 4 hours, and the excess thionyl chloride was removed by rotary evaporation to obtain a pale yellow oil, which was dissolved in dry ether ( 10 mL), filtered to remove insoluble matter, added dry triethylamine (2 mL, 14.3 mmol), then added p-toluidine (565 mg, 5.28 mmol), and stirred the reaction solution at room temperature for 5 hours. After the reaction, add 20 mL of 10% NaOH aqueous solution, extract with dichloromethane (3 times, 20 mL each time), combine the organic phases, wash with saturated brine, anhydrous MgSO 4 After drying, the solvent was evaporated under vacuum, and methanol was used as developing solvent to separate the pure product with a yield of 68.5%. M.p.: 169-170℃, IR(KBr): v3030, 2923, 2866, 1618, 1587, 1513, 1486, 1420, 1383, 1322,...
example 2
[0018] Example 2, the preparation of 1,3-bis((S)-4-isopropyl-4,5-dihydro-1-p-tolyl-1H-imidazol-2-yl)benzene: N,N'-bis A solution of ((S)-1-hydroxy-3-methylbutyl-2-yl)-1,3-dibenzamide (2.4 mmol) in thionyl chloride (3 mL, 41.3 mmol) was stirred at reflux for 6 hours, The excess thionyl chloride was rotary evaporated to obtain a pale yellow oil, which was dissolved in dry diethyl ether (10 mL), filtered to remove insoluble matter, and dried triethylamine (2 mL, 14.3 mmol) was added, followed by p-toluidine (565 mg, 5.28 mmol), and the reaction solution was stirred at room temperature for 6 hours. After the reaction, 20 mL of 10% NaOH aqueous solution was added, extracted with dichloromethane (3 times, 20 mL each time), the organic phases were combined, washed with saturated brine, anhydrous MgSO 4 After drying, the solvent was evaporated under vacuum, and acetone was used as a developing agent to separate the product with a yield of 44.5%. Pale yellow oil, IR (KBr): v 3032, 29...
example 3
[0019] Example 3, the preparation of 1,3-bis((S)-4-benzyl-4,5-dihydro-1-p-tolyl-1H-imidazol-2-yl)benzene: N,N'-bis( (S)-1-hydroxy-3-phenylpropyl-2-yl)-1,3-dibenzamide (2.4mmol) in thionyl chloride (3mL, 41.3mmol) was stirred under reflux for 8 hours, and The excess thionyl chloride was distilled off to obtain a pale yellow oil, which was dissolved in dry diethyl ether (10 mL), filtered to remove insoluble matter, and dried triethylamine (2 mL, 14.3 mmol) was added, followed by p-toluidine ( 565mg, 5.28mmol), and the reaction solution was stirred at room temperature for 7 hours. After the reaction, 20 mL of 10% NaOH aqueous solution was added, extracted with dichloromethane (3 times, 20 mL each time), the organic phases were combined, washed with saturated brine, anhydrous MgSO 4 After drying, the solvent was evaporated under vacuum, and acetone was used as a developing agent to separate the product with a yield of 53.7%. Pale yellow oil, IR (KBr): v3060, 3027, 2922, 2867, 16...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com