Method for separating and purifying recombined hepatitis b surface antigen expressed by Hansenula yeast
A technology of hepatitis B surface antigen and Hansenula, which is applied in the field of protein separation and purification, can solve the problems of low activity recovery rate, long cycle, and many steps of HBsAg, and achieve the effect of simple production process, short operation cycle, and great practical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1, laboratory scale separation and purification experiment 1
[0036] Take 200ml of the culture supernatant of recombinant HBsAg expressed by Hansenula spp. obtained by conventional process fermentation culture, first centrifuge and wash the Hansenula saccharomyces cells used to express HBsAg, then place it in a high-pressure homogenizer, add The weight ratio of the crushing liquid is 0.05% Triton X-100 and 1 mM PMSF, and the cell crushing rate reaches 90% after repeated crushing for 6 times.
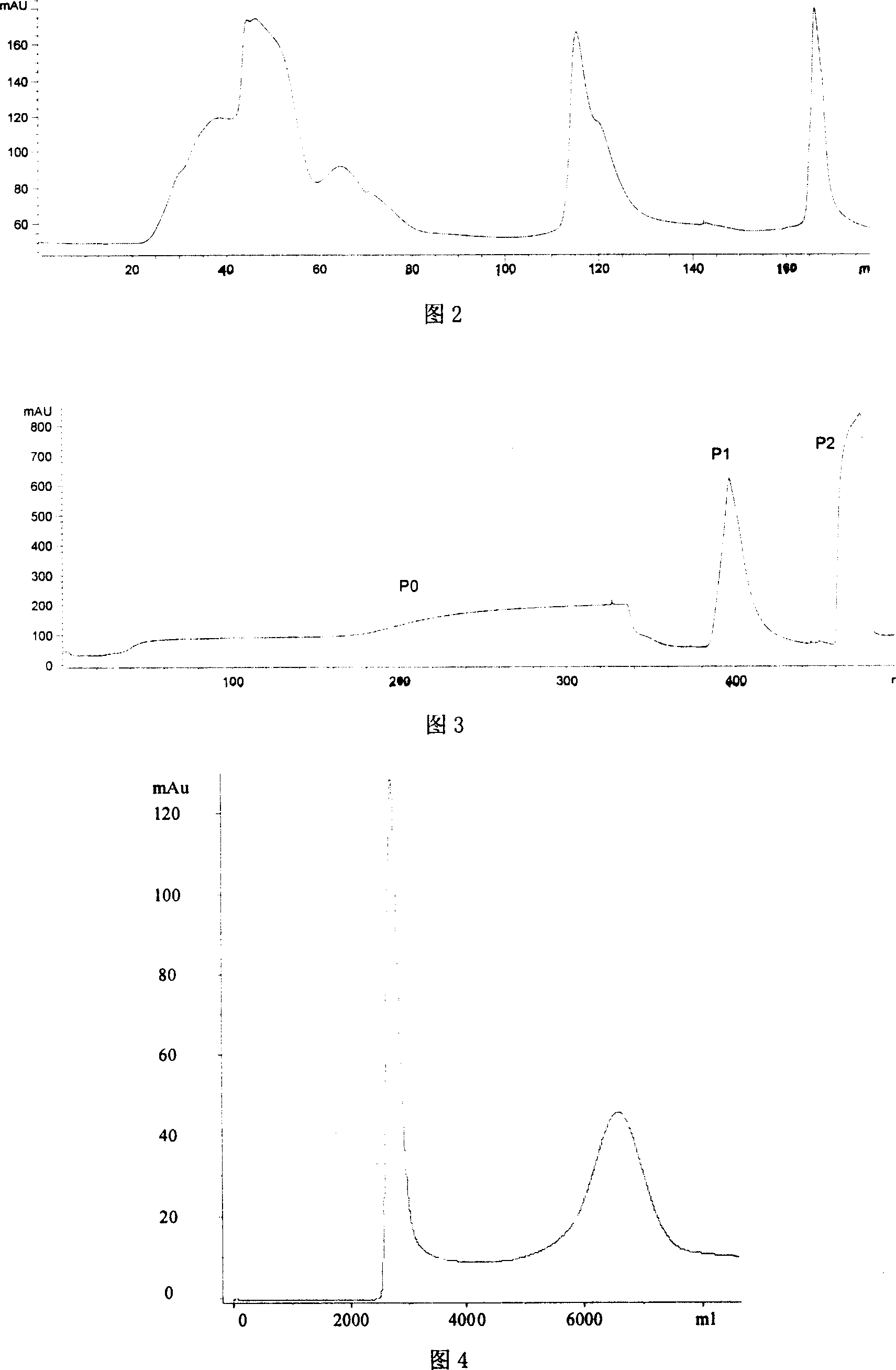
[0037]The broken cells were centrifuged at 20000g for 20min to remove cell debris, the supernatant was adjusted to pH 5.5 with 1.0M NaOH and 1.0M HCl, and the conductivity was less than 5.0mS / cm, and then fed to Q SepharoseFF ion exchange In a chromatography column (GE Healthcare, 30cm×15cm I.D.), the column volume (CV) is 2000ml, and the flow rate is 1.0ml / (h·ml gel). The chromatography process is followed by buffer I (20mM phosphate buffer (PB), pH5.5) equilibration...
Embodiment 2
[0041] Embodiment 2, laboratory scale separation and purification experiment 2
[0042] Take 200ml of the culture supernatant of recombinant HBsAg expressed by Hansenula spp. obtained by conventional fermentation and culture, first centrifuge and wash the Hansenula saccharomyces cells used to express HBsAg, then place it on a ball mill, and add the broken solution The weight ratio of 0.3% Triton X-100 and 0.1mM PMSF was repeatedly crushed twice, and the cell crushing rate reached 50%.
[0043] The broken cells were centrifuged at 6000g for 30min to remove cell debris, the supernatant was adjusted to pH 8.5 with 1.0M NaOH and 1.0M HCl, and the conductivity was less than 20mS / cm, and then fed to the Q SepharoseFF ion exchange layer In a column (GE Healthcare, 30cm×15cm I.D.), the column volume (CV) is 2000ml, and the flow rate is 20ml / (h·ml gel). The chromatography process is followed by buffer I (20mM Tris-HCl buffer, pH8.5) equilibration, feeding, buffer I rebalance, 0-100% b...
Embodiment 3
[0047] Embodiment 3, laboratory scale separation and purification experiment 3
[0048] Take 50ml of the culture supernatant of recombinant HBsAg expressed by Hansenula spp. obtained by conventional fermentation and culture, first centrifuge and wash the Hansenula saccharomyces cells used to express HBsAg, then place it on a high-pressure homogenizer, and add The weight ratio of the disrupting fluid is 0.1% Triton X-100 and 10mM PMSF, and the disrupting solution is repeated 3 times, and the cell disrupting rate reaches 70%.
[0049] The broken cells were centrifuged at 10000g for 20min to remove cell debris, the supernatant was adjusted to pH 7.0 with 1.0M NaOH and 1.0M HCl, and the conductivity was less than 10mS / cm, and then fed to the DEAE QZTFF ion exchange layer In the analysis column (National Biochemical Engineering Center, 30cm×5.5cm I.D.), the column volume (CV) is 500ml, and the flow rate is 10ml / (h·ml gel). The chromatographic process is followed by buffer I (20mM ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com