Penciclovir freeze dried and method of manufacturing the same
A technology of freeze-dried powder injection and penciclovir, which is applied in the field of medicine to achieve the effect of ensuring safety and convenience, no shrinkage, and good molding of the finished product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
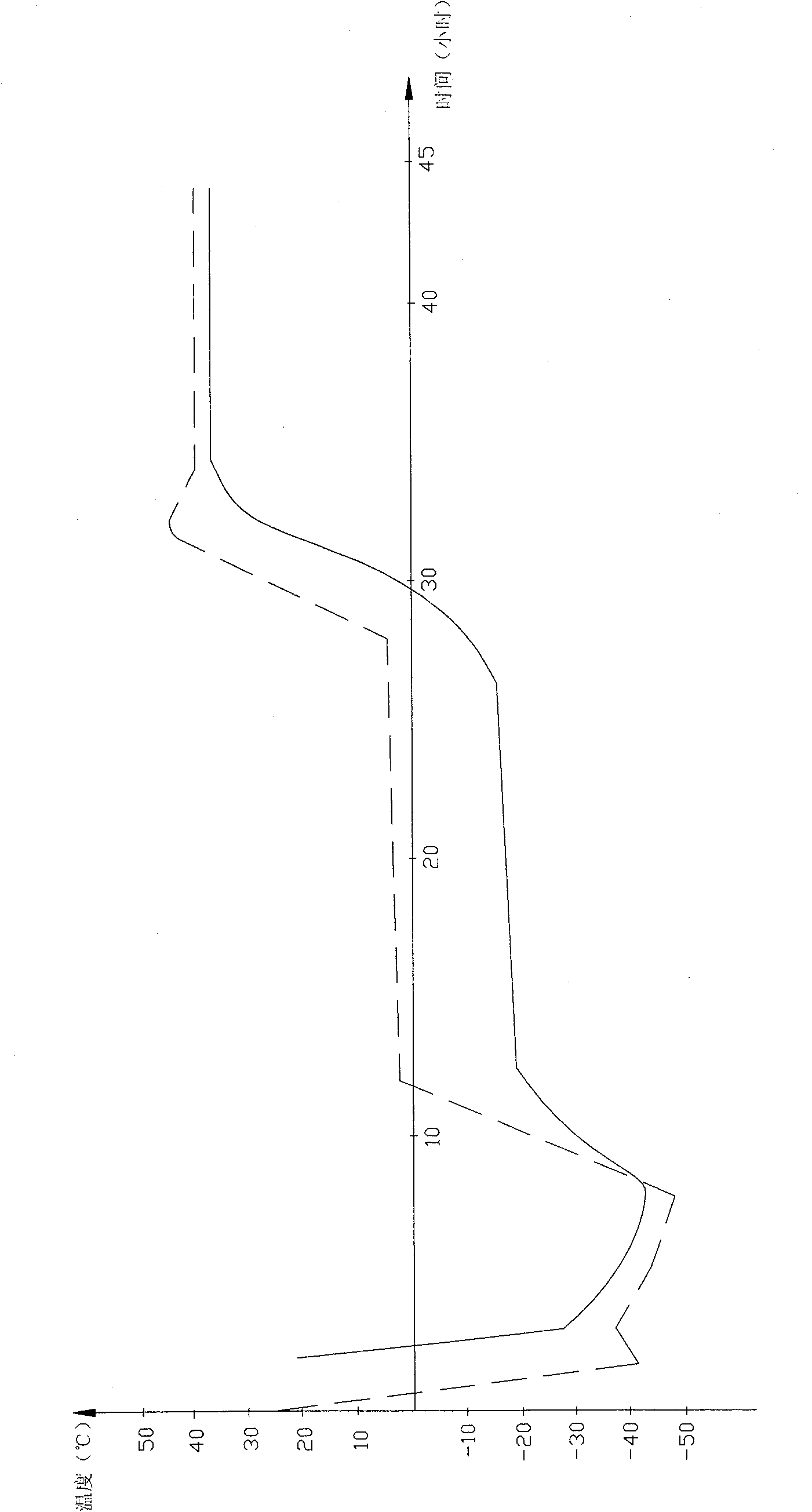
[0027] Get 250g of penciclovir, dissolve with 5% sodium hydroxide solution until clarification (control pH value 11.0); 100g of low-molecular-weight d-glucoside is prepared into a 10% solution with water for injection, add 0.5% activated carbon, and boil for 30min , cooling and filtering. Mix the above two solutions, add 0.5% activated carbon, stir for 30 minutes, filter, take a sample to measure the content, add water for injection to the specified volume, and measure the pH value. Subpackaged products are put into the freeze dryer and freeze-dried (the freeze-drying curve is as follows: figure 1 As shown), pre-freeze for 3 hours after entering the box, the shelf temperature is -40°C, the product temperature is -25°C, the vacuum pump is turned on and the product begins to sublimate, and the product temperature is controlled at -18°C during the sublimation stage (the eutectic point of the product is about -9 ℃), after the water line reaches the bottom, the temperature of the ...
Embodiment 2
[0029] Get 300g of penciclovir, dissolve with 5% sodium hydroxide solution to clarification (control pH value 11.0); 120g of low-molecular-weight d-glucoside, first prepare 10% solution with water for injection, add 0.5% active carbon, boil for 30min , cooling and filtering. Mix the above two solutions, add 0.5% activated carbon, stir for 30 minutes, filter, take a sample to measure the content, add water for injection to the specified volume, and measure the pH value. Put the packaged products into the freeze dryer for freeze-drying, and pre-freeze for 4 hours after entering the box. The temperature of the shelf is -45°C, and the temperature of the product is -25°C. Turn on the vacuum pump and the product starts to sublimate. 20°C (the eutectic point of the product is about -9°C), after the water line reaches the bottom, the temperature of the product slowly rises to about 37°C, the temperature of the shelf is 40°C, keep it warm for 10 hours, and press the plug out of the box...
Embodiment 3
[0031] Get 400g of penciclovir, dissolve with 5% sodium hydroxide solution until clarification (controlling pH value 11.0); 160g of low-molecular-weight d-glucoside is prepared into a 10% solution with water for injection, add 0.5% activated carbon, and boil for 30min , cooling and filtering. Mix the above two solutions, add 0.5% activated carbon, stir for 30 minutes, filter, take a sample to measure the content, add water for injection to the specified volume, and measure the pH value. Put the packaged products into the freeze dryer for freeze-drying, and pre-freeze for 5 hours after entering the box. The shelf temperature is -50°C, the product temperature is -30°C, and the vacuum pump is turned on. The product starts to sublime, and the product temperature is controlled at - 20°C (the eutectic point of the product is about -9°C), after the water line reaches the bottom, the temperature of the product slowly rises to about 37°C, the temperature of the shelf is 41°C, keep it w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com