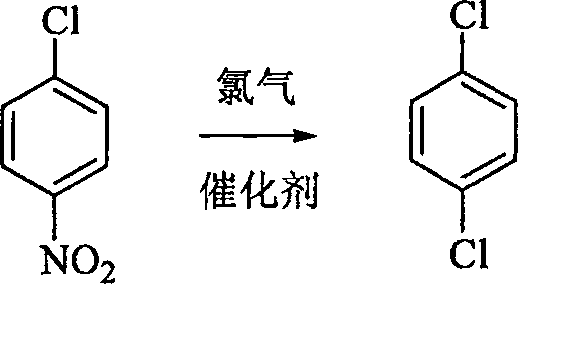
Method for synthesizing p-dichlorobenzene
A technology of p-dichlorobenzene and p-nitrochlorobenzene, which is applied in the field of synthesis of p-dichlorobenzene, can solve the problems of small scale, difficult separation, and poor product quality, and achieve mild reaction conditions, simple process operation, and simple equipment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 15.8 grams (0.1 moles) of p-nitrochlorobenzene and 0.08 grams of catalyst benzoyl peroxide into the reaction flask, heat to 200 ° C, and feed chlorine gas to react for 6 hours to obtain the crude product of p-dichlorobenzene, which is rectified Column rectification, obtains the product 13.6 grams of purity ≥ 99.5%, yield 93%. The melting point is 52-53°C. The brown-red gas (nitroxyl chloride) produced during the reaction is absorbed with ammonia and ammonium chloride liquid.
Embodiment 2
[0024] Add 15.8 grams (0.1 moles) of p-nitrochlorobenzene and 0.08 grams of catalyst azobisisobutyronitrile into the reaction flask, heat to 200 ° C, and feed chlorine gas to react for 6 hours to obtain the crude product of p-dichlorobenzene. Distillation column rectification, obtains the product 13.8 grams of purity ≥ 99.5%, yield 94%. The melting point is 52-53°C. The brown-red gas (nitroxyl chloride) produced during the reaction is absorbed with ammonia and ammonium chloride liquid.
Embodiment 3
[0026] Add 15.8 g (0.1 mole) of p-nitrochlorobenzene and 0.1 g of catalyst azobisisobutyronitrile into the reaction flask, heat to 150° C., and feed chlorine gas to react for 6 hours to obtain the crude p-dichlorobenzene, which is refined and purified. Distillation column rectification, obtains the product 13 grams of purity ≥ 99.5%, yield 89%. The melting point is 52-53°C. The brown-red gas (nitroxyl chloride) produced during the reaction is absorbed with ammonia and ammonium chloride liquid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com