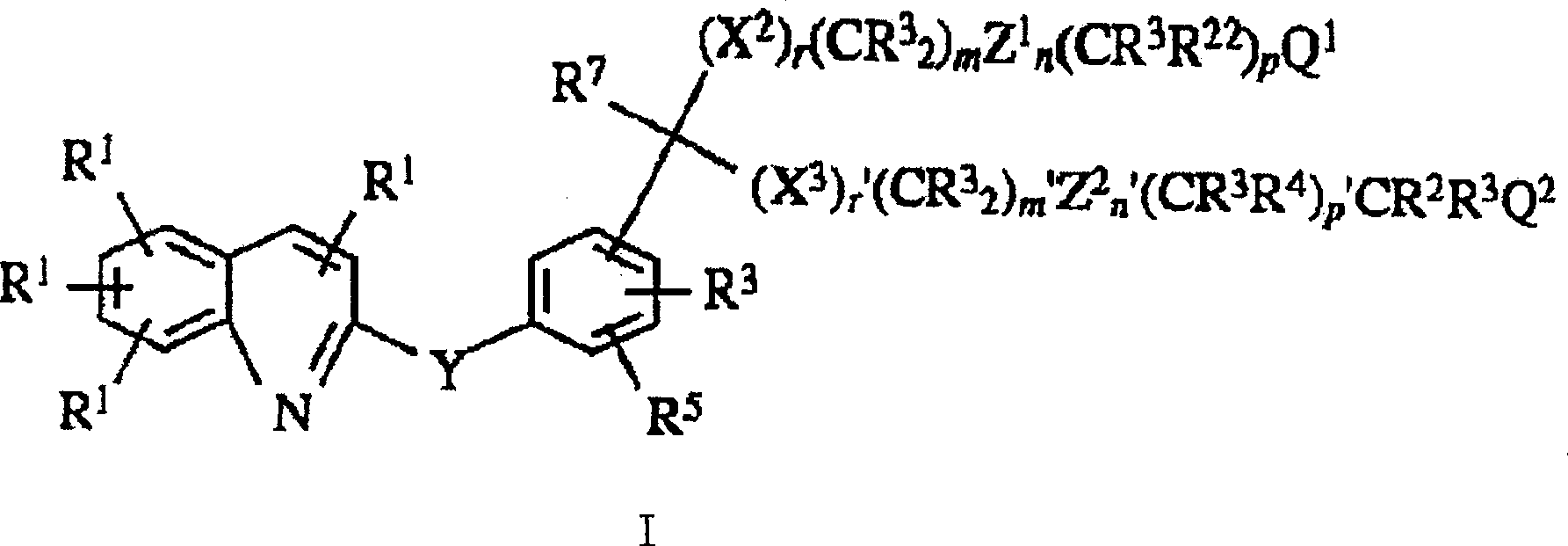
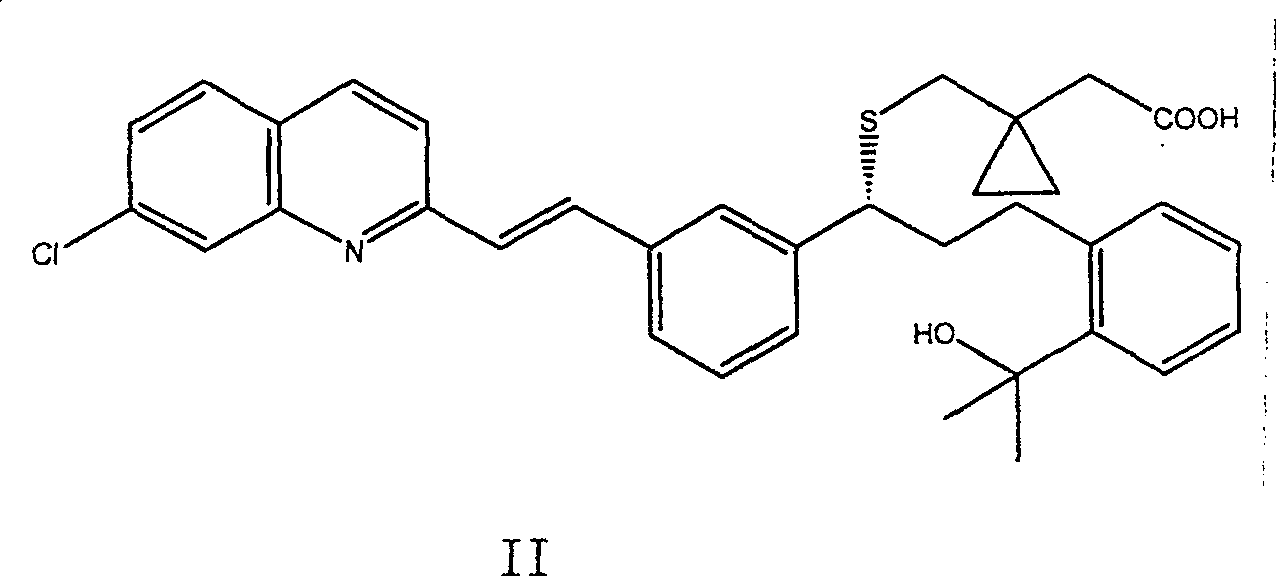
Composition of leukotrienes antagonist oral liquid
A technology of oral liquid and composition, which is applied in the field of anti-allergic oral liquid composition, and can solve problems such as difficulty in swallowing pills
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Dissolve Montelukast sodium salt in propylene glycol, dissolve dipotassium hydrogen phosphate, potassium dihydrogen phosphate, sodium saccharin, sodium benzoate, food coloring and spices in water, adjust the concentration of the aqueous solution with 1N sodium hydroxide aqueous solution pH value to pH9~10. After mixing the aqueous phase and the organic phase, the pH value of the solution was adjusted to pH 9-10 with 1N sodium hydroxide aqueous solution, and the concentration of Montelukast sodium salt was 0.2% w / v, which was completely dissolved. Stability tests were performed at 40°C and 60°C, respectively. After storage at 40°C for 150 days, the concentration of Montelukast sodium salt in the solution did not change; after storage at 60°C for 120 days, the concentration of Montelukast sodium salt in the solution did not change either.
[0032] The concentration of the phosphate-containing component in the aqueous solution is about 0.5-7% w / v. The concentration of th...
Embodiment 2
[0034] Dissolve Montelukast sodium salt in propylene glycol, dipotassium hydrogen phosphate, potassium dihydrogen phosphate, sodium saccharin, sodium benzoate, Tween 80, food coloring and spices in water, and add 1N sodium hydroxide Aqueous solution Adjust the pH value of the aqueous solution to pH9-10. After mixing the aqueous phase and the organic phase, the pH value of the solution was adjusted to pH 9-10 with 1N sodium hydroxide aqueous solution, and the concentration of Montelukast sodium salt was 0.2% w / v, which was completely dissolved. Stability tests were performed at 40°C and 60°C, respectively. After storage at 40°C for 150 days, the concentration of Montelukast sodium salt in the solution did not change; after storage at 60°C for 120 days, the concentration of Montelukast sodium salt in the solution did not change either.
[0035] The concentration of the phosphate-containing component in the aqueous solution is about 0.5-7% w / v. In this embodiment, the concentra...
Embodiment 3
[0037] Dissolve Montelukast sodium salt in propylene glycol, dissolve boric acid, potassium chloride, saccharin sodium salt, sodium benzoate, food coloring and spices in water, and adjust the pH value of the aqueous solution to pH9~10. After mixing the aqueous phase and the organic phase, the pH value of the solution was adjusted to pH 9-10 with 1N aqueous sodium hydroxide solution, and the concentration of Montelukast sodium salt was 0.1% w / v, which was completely dissolved. Stability tests were performed at 40°C and 60°C, respectively. After storage at 40°C for 45 days, the concentration of Montelukast sodium salt in the solution did not change; after storage at 60°C for 45 days, the concentration of Montelukast sodium salt in the solution did not change either.
[0038] The concentration of the borate-containing component in the aqueous solution is about 0.1-3% w / v. The concentration of the borate-containing component in this example is about 0.31% w / v. And the consumpti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com