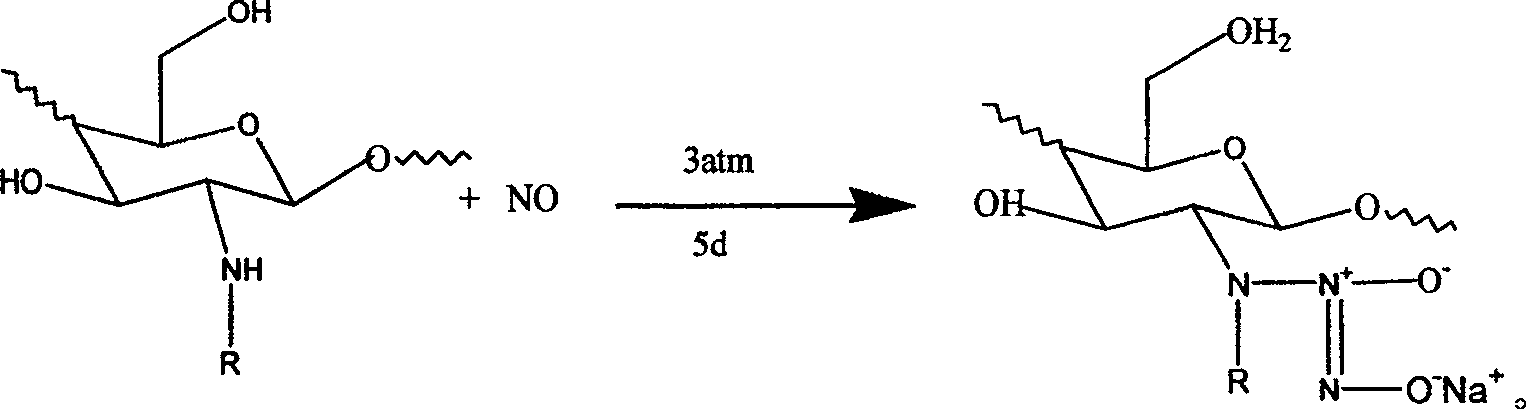
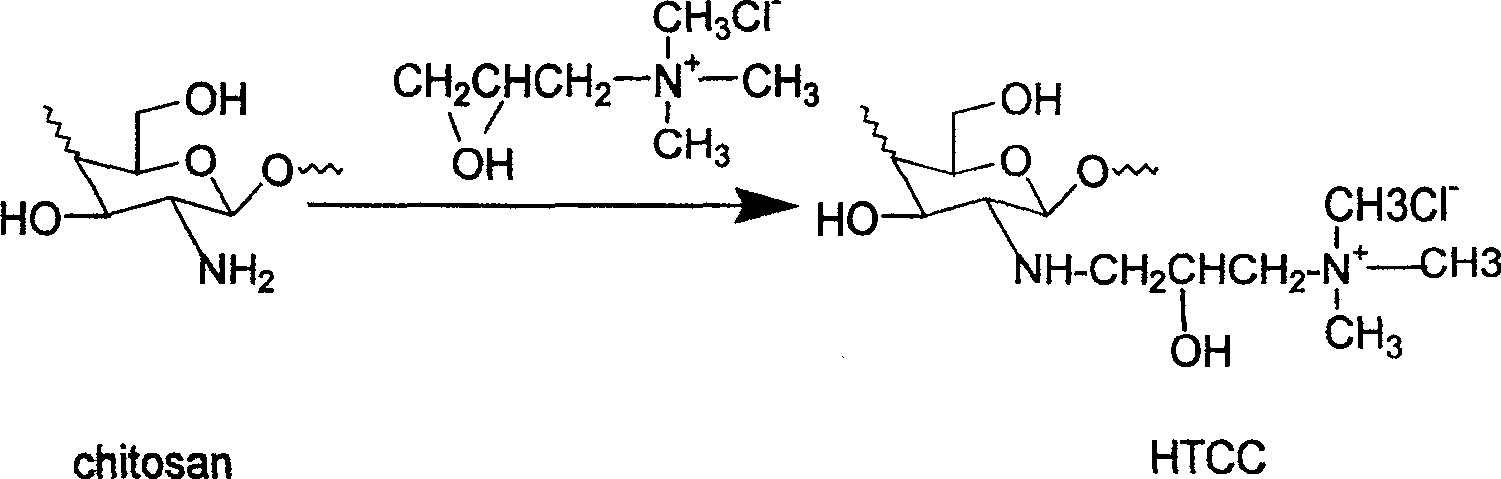
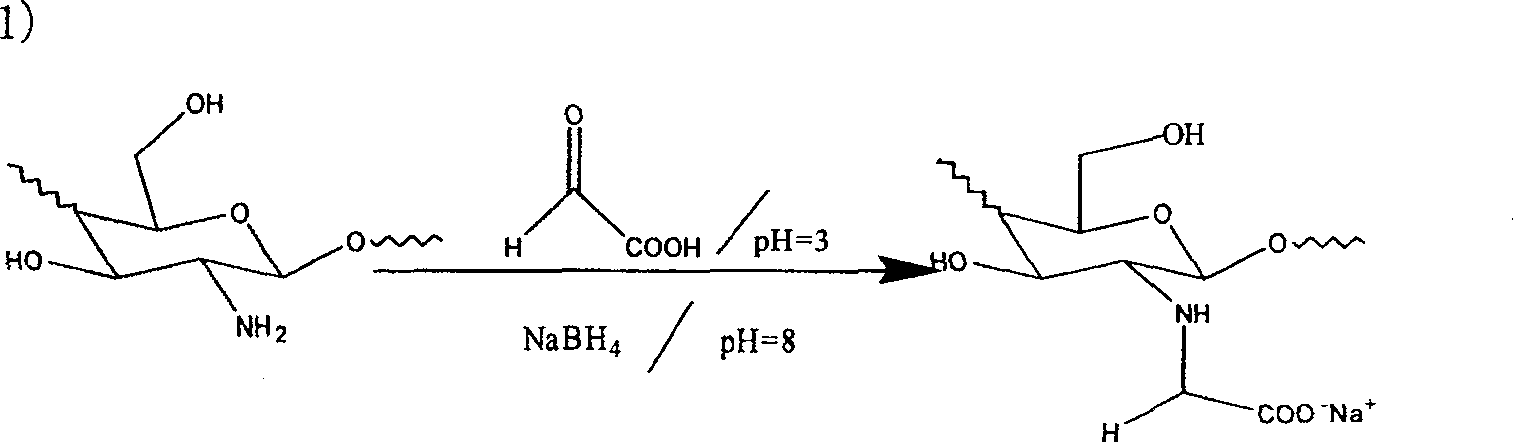
Synthesis of quaternary ammonium salt modified nucleophilic NO donor
A synthesis method and quaternary ammonium modification technology, which is applied in the field of medical engineering, can solve the problems that nucleophilic NO donors cannot be directly targeted and cannot react in a homogeneous phase, so as to improve antithrombotic performance, stabilize chemical properties, and prevent recurrence. narrow effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This embodiment 1 is implemented under the following conditions of implementation and technical requirements:
[0034](1) Synthesis of N-monoquaternary ammonium chitosan (HTCC): Chitosan (6g, 37.0mmol) was dissolved in deionized water (60ml), and GTMAC (7.1ml, 111mmol) was dissolved at a reaction temperature of 85°C. It was added to the above reaction solution three times at intervals of two hours. After reacting for 10 hours, the transparent yellow reaction solution was added to ice acetone (200ml) solution, stirred and placed in the refrigerator overnight. The next day, acetone was discarded and ethanol was added to dissolve, and then precipitated with 4:1 acetone-ethanol solution, pumped and washed, and the final product was dried in a vacuum oven at 60°C to obtain 10.23 g of light yellow powder.
[0035] (2) Synthesis of quaternary ammonium salt modified chitosan / NO donor: 1.2g (0.004mol) of quaternary ammonium salt modified chitosan was added to 100ml of anhydrous...
Embodiment 2
[0038] This embodiment 2 is implemented under the following conditions of implementation and technical requirements:
[0039] (1) Synthesis of N-monoquaternary ammonium chitosan (HTCC): Chitosan (6g, 37.0mmol) was dissolved in deionized water (60ml), and GTMAC (7.1ml, 111mmol) was dissolved at a reaction temperature of 85°C. It was added to the above reaction solution three times at intervals of two hours. After reacting for 17 hours, the transparent yellow reaction solution was added to a solution of ice acetone (200 ml), stirred and placed in the refrigerator overnight. The next day, acetone was discarded and ethanol was added to dissolve, and then precipitated with 4:1 acetone-ethanol solution, pumped and washed, and the final product was dried in a vacuum oven at 60°C to obtain 10.72 g of light yellow powder.
[0040] (2) Synthesis of quaternary ammonium salt modified chitosan / NO donor: 1.2g (0.004mol) of quaternary ammonium salt modified chitosan was added to 100ml of an...
Embodiment 3
[0043] This embodiment 3 is implemented under the following conditions of implementation and technical requirements:
[0044] (1) Synthesis of N-monoquaternary ammonium chitosan (HTCC): Chitosan (6g, 37.0mmol) was dissolved in deionized water (60ml), and GTMAC (7.1ml, 111mmol) was dissolved at a reaction temperature of 85°C. It was added to the above reaction solution three times at intervals of two hours. After 24 hours of reaction, the transparent yellow reaction solution was added to a solution of ice acetone (200 ml), stirred and placed in the refrigerator overnight. The next day, acetone was discarded and ethanol was added to dissolve, and then precipitated with 4:1 acetone-ethanol solution, pumped and washed, and the final product was dried in a vacuum oven at 60°C to obtain 11.02 g of yellow powder.
[0045] (2) Synthesis of quaternary ammonium salt modified chitosan / NO donor: 1.2g (0.004mol) of quaternary ammonium salt modified chitosan was added to 100ml of anhydrous...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com