Preparation method of breviscapine raw material medicine by using big-hole resin
A macroporous resin, breviscapine technology, which is applied in the directions of sugar derivatives, ion exchange regeneration, ion exchange, etc., can solve problems such as not being well solved, and achieves the problem of solving refining problems, simple process steps, and easy industrial production. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
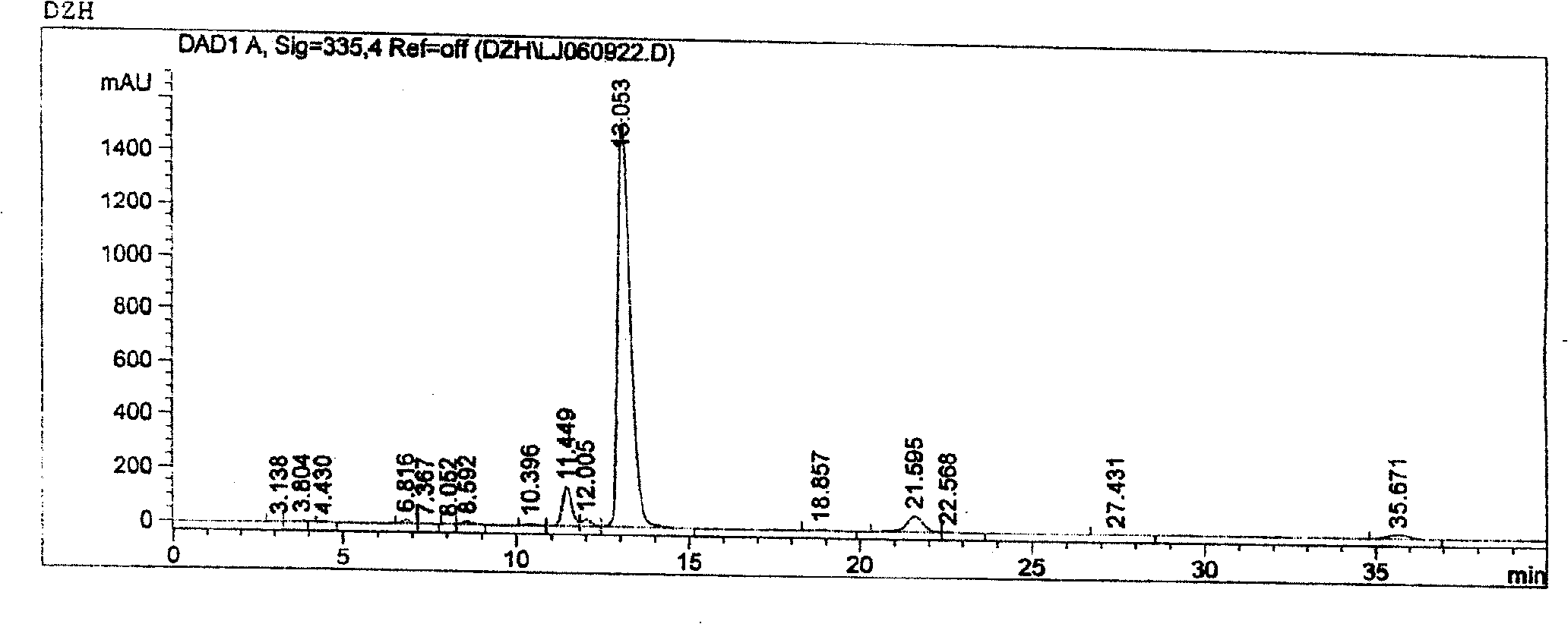
[0027] Weigh 500g of scutellarin raw material with a scutellarin B content of 84.69%, add boiling water to prepare a concentration of 20% by weight, adjust the pH value to 7.5 with 20% sodium bicarbonate, and dissolve it. The filtrate is first passed through a 16000rpm continuous tube After centrifugation in a test centrifuge, filter again, add the filtrate to the D101 macroporous resin column, use water as the eluent, and collect the eluate until it is basically colorless. Use 20% hydrochloric acid to adjust the pH value of the eluent to 1, let it settle for 10 hours, filter, wash the precipitate with water until neutral (use AgNO3 to check for no Cl-), dry it at 105°C, send it for inspection, and pass through the HPLC layer According to the analysis and analysis, the content of breviscapine raw material medicine is increased from 84.69% to 91.65% from the original B content, which meets the requirements of "Drug Standards" breviscapine injection (1998) issued by the Ministry....
Embodiment 2
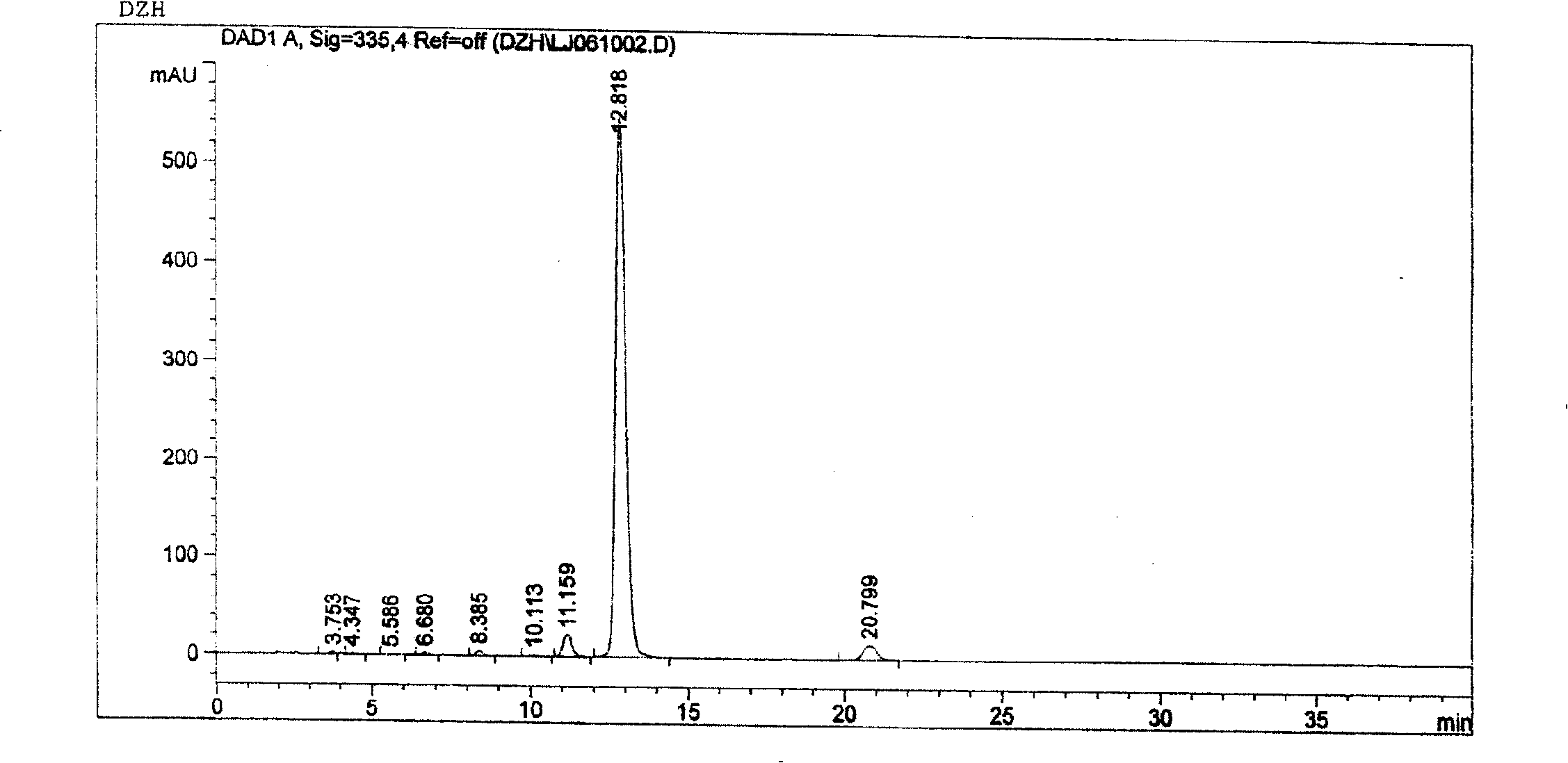
[0068] Weigh 500g of scutellarin raw material with a scutellarin B content of 78.99%, add boiling water to prepare a concentration of 10% by weight, adjust the pH value to 7.5 with 5% sodium hydroxide solution, and dissolve the filtrate at 16000rpm After centrifugation in a continuous tube test centrifuge, filter again, add the filtrate to the AB-8 macroporous resin column, use water as the eluent, and collect the eluate until it is basically colorless. The eluate was adjusted to pH 1 with 15% sulfuric acid solution, allowed to settle for 10 hours, filtered, and the precipitate was washed with water until neutral (use AgNO3 to check for the absence of Cl-). All operations are repeated 1 time. Dried at 80°C, sent for inspection, and analyzed by high performance liquid chromatography, the content of breviscapine raw material drug increased from 78.99% to 91.99%, which complied with the "Drug Standard" issued by the Ministry of Breviscapine Injection (1998 )Regulation.
[0069]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com