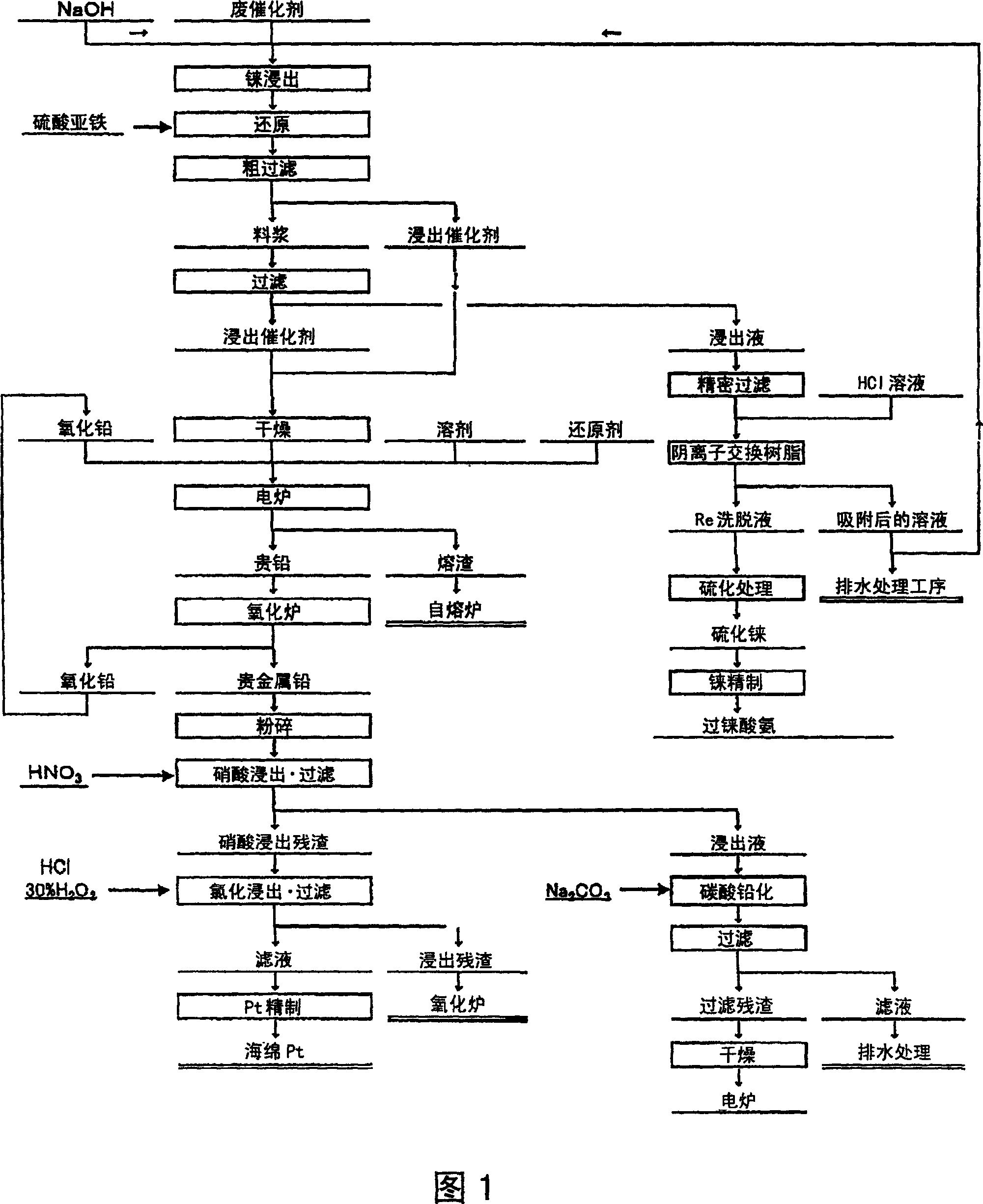
Method of recovering platinum and rhenium from waste catalyst
A technology of waste catalyst and rhenium sulfide, applied in the field of recovering platinum and rhenium, can solve the problems of low recovery rate, dilution of platinum concentration, low recovery rate of rhenium, etc., and achieve the effect of high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] 15g of the spent catalyst containing 0.22mass% platinum and 0.43mass% rhenium loaded on a porous carrier containing alumina was used in 150mL of various leaching solutions of various concentrations under the temperature conditions from room temperature to 90°C, After leaching with a reaction time of 30 minutes to 120 minutes, filter, measure the amount of filtrate, analyze the filtrate, obtain the leaching rate of rhenium, and obtain the results in Table 1.
[0054] The leaching rate of rhenium is high in alkaline solution, especially sodium carbonate (Na 2 CO 3 ) or sodium hydroxide (NaOH) solution.
[0055] As for the leaching temperature conditions, the higher the temperature under any condition, the better. When the temperature is above 90°C, the leaching rate of rhenium above 90% can be obtained.
[0056] In 4mass% NaOH solution, when the reaction time is more than 30 minutes, the rhenium leaching rate of more than 90% can be obtained.
[0057] Table 1 Relations...
Embodiment 2
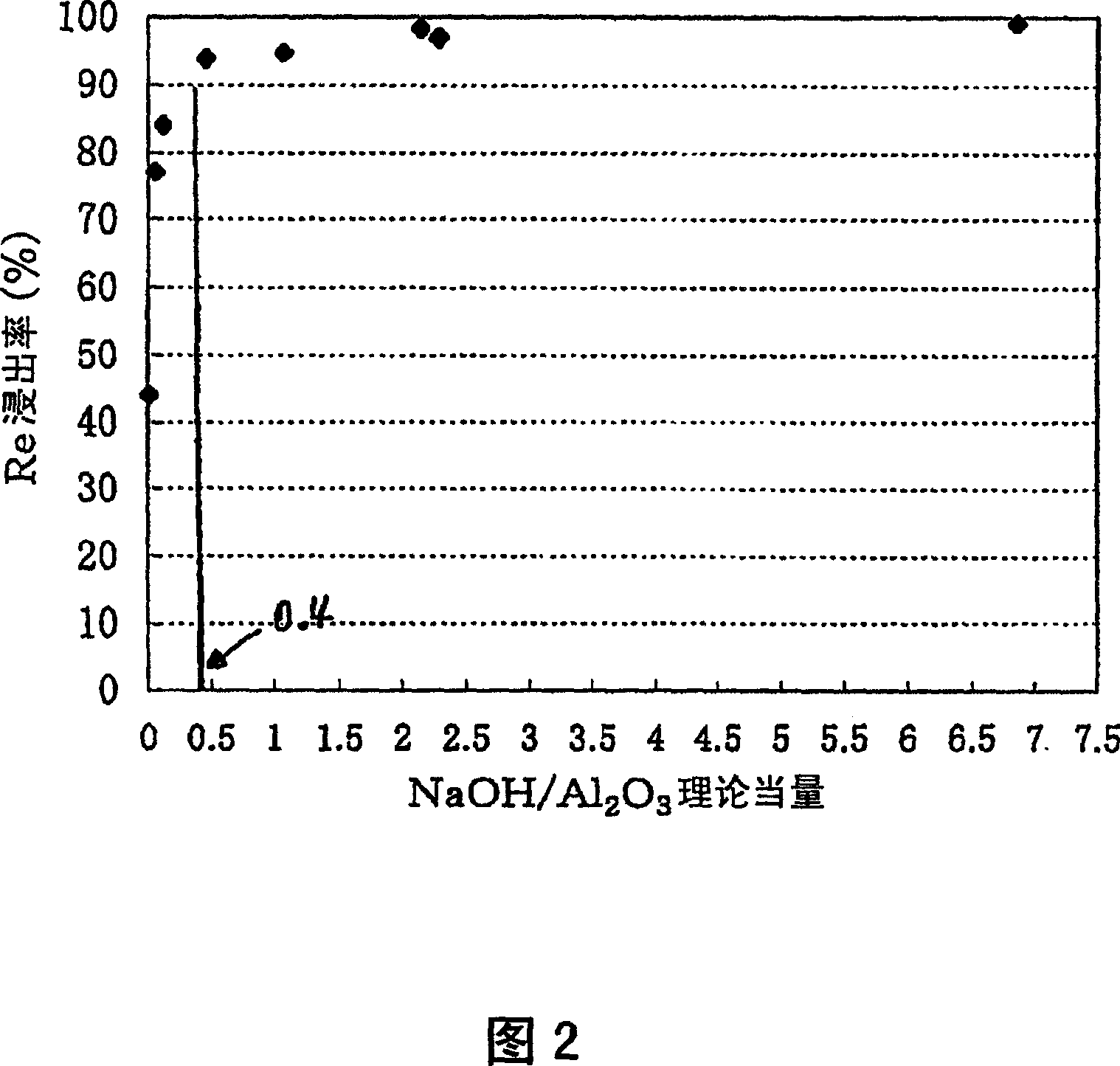
[0061] In the rhenium leaching process, in order to grasp the relationship between the rhenium leaching rate and the concentration of the NaOH solution, 15 g of the spent catalyst was put into NaOH / (Re+Pt+Al 2 o 3 )=0.006~6.9 in the NaOH solution 150mL of the various concentrations of equivalent range, filter after leaching 1 hour, after measuring the filtrate amount, analyze the filtrate, obtain the leaching rate of each component, thereby obtain the result of Fig. 2. If more than 0.4 times of the theoretical amount of NaOH is added, the leaching rate of rhenium will be more than 90%.
Embodiment 3
[0063] Put 220 g of spent catalyst containing 0.22 mass% platinum and 0.43 mass% rhenium loaded on a porous carrier containing alumina into 1.1 L of a NaOH concentration solution equivalent to 0.4 times the theoretical amount, and leach rhenium at a temperature of 90°C After 1 hour, solid-liquid separation was carried out, and the platinum concentration in the filtrate was analyzed.
[0064] The platinum concentration in the filtrate was 64 mg / L, and the rhenium concentration was 800 mg / L. Get 5 parts of 200mL described filtrate respectively, be heated to 90 ℃, add the concentration of each condition required amount in this solution again and be the ferrous sulfate (FeSO4 of 43g / L) 4 ·7H 2 (2) solution, and carry out after 10 minutes reaction, filter, the platinum in filtrate, rhenium concentration are analyzed, obtain the result of table 2.
[0065] If more than 1.5 times the theoretical amount of the ferrous sulfate solution required for platinum reduction is added, the pl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com