Methods for high yield production of terpenes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Experimental Procedures
[0312]Plant Materials:
[0313]Wild-type Camelina sativa was grown in the green house at Donald Danforth Plant Science Center. Peppermint Mentha piperita leaves were harvested from a garden in St. Louis, Mo. in September, 2009.
[0314]Cloning of Peppermint Geranyl Diphosphate Synthase and Limonene Synthase cDNAs
[0315]Total RNA was extracted from peppermint leaves using RNeasy plant mini kit (Qiagen). First-strand cDNAs was synthesized using SuperScript III (Invitrogen). References and nucleotide sequences (Burke et al. (1999) Arch. Biochem. Biophys., 422, 52-60; Alonso et al. (1992) J Biol Chem., 267, 7582-7; Colby et al. (1993) J Biol Chem., 268, 23016-24. NCBI accession numbers: AF182827, AJ249453, EU108697, AW255818) were used to design cloning primers. Geranyl diphosphate synthase small subunit without predicted chloroplast transit peptide has been cloned from the peppermint cDNAs with primers: GSSfC and GSSr4 (Table E1) (FIG. 10). Geranyl diphosphate synthase ...
example 2
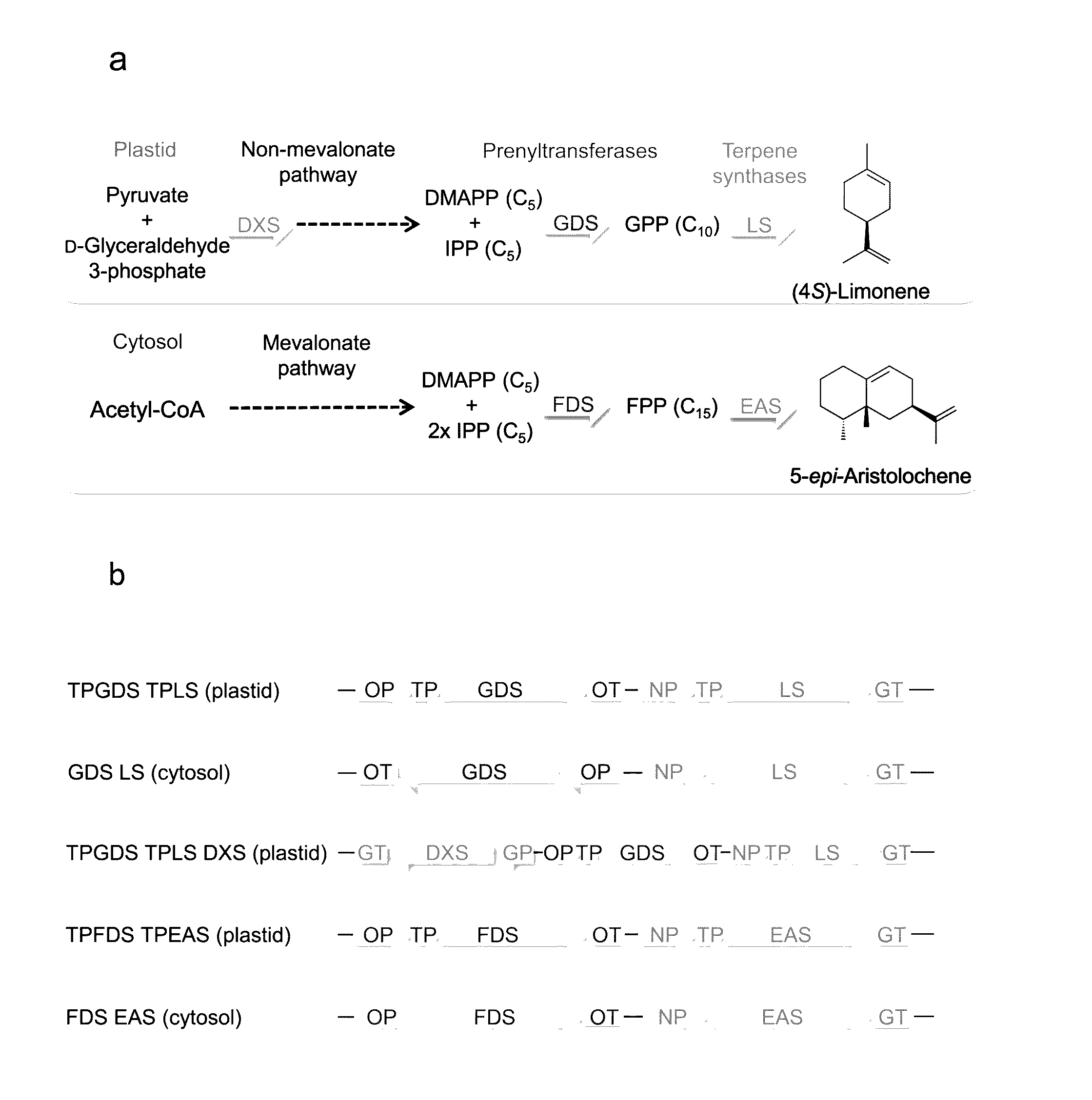
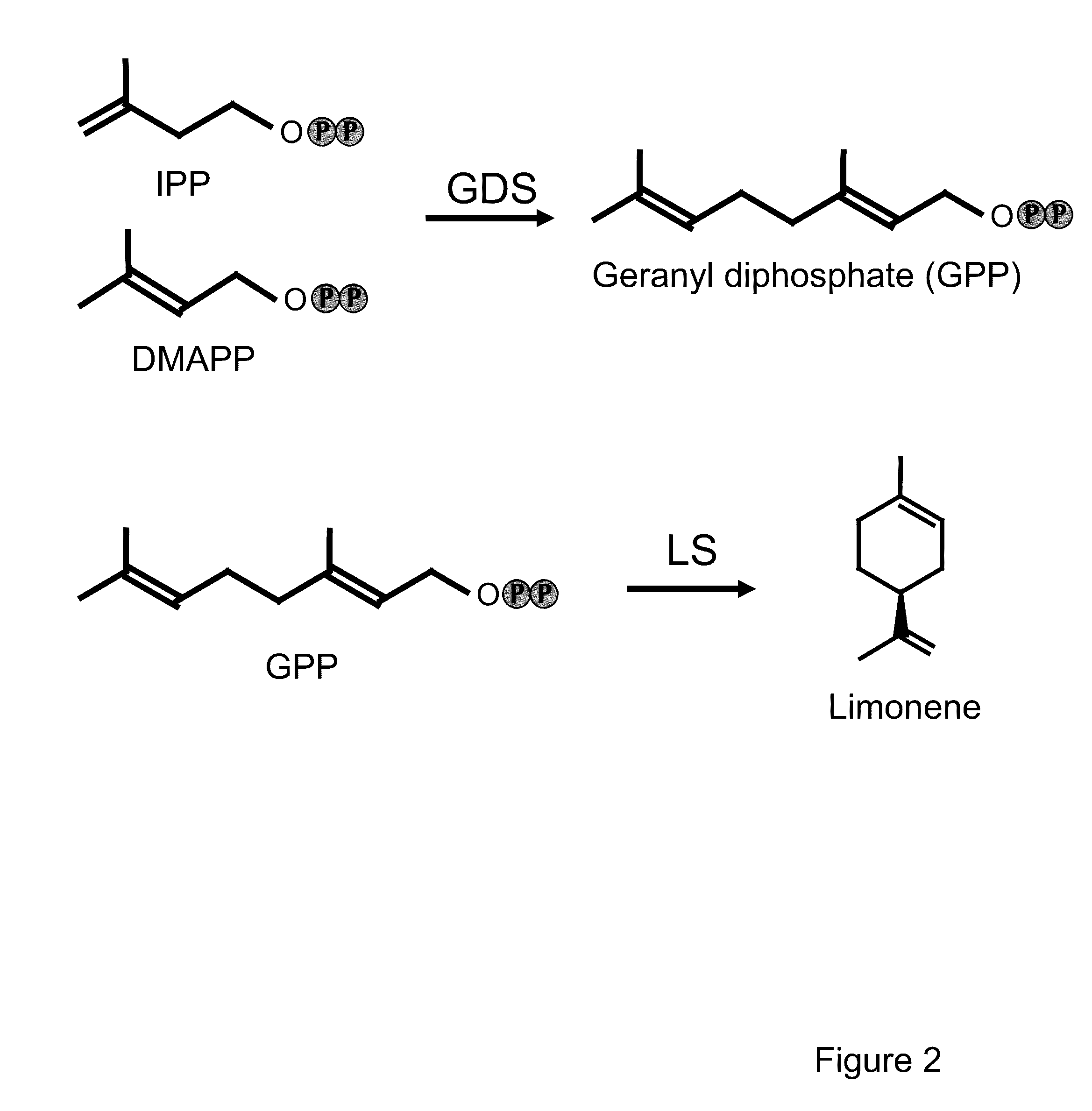
Biosynthesis and Accumulation of Cyclic Monoterpene Hydrocarbon (4S)-Limonene and the Bicyclic Sesquiterpene Hydrocarbon 5-Epi-Aristolochene in Camelina Seed
[0330]This example describes the biosynthesis and accumulation of the cyclic monoterpene hydrocarbon (4S)-limonene and the bicyclic sesquiterpene hydrocarbon 5-epi-aristolochene in camelina seed by expressing appropriate combinations of terpene biosynthetic enzymes.
[0331]The phrase “biosynthetically appropriate combination of enzymes” refers to a combination of terpene biosynthetic enzymes that facilitates the biosynthesis of a monoterpene or sesquiterpene of interest. Such combinations include a combination of: 1) a geranyl diphosphate synthase and a monoterpene synthase that catalyzes the formation of a monoterpene of interest, or 2) a combination of a farnesyl diphosphate synthase and a sesquiterpene synthase that catalyzes the formation of a sesquiterprene of interest. The phrase “a biosynthetically appropriate combination o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| retention time | aaaaa | aaaaa |
| retention time | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com