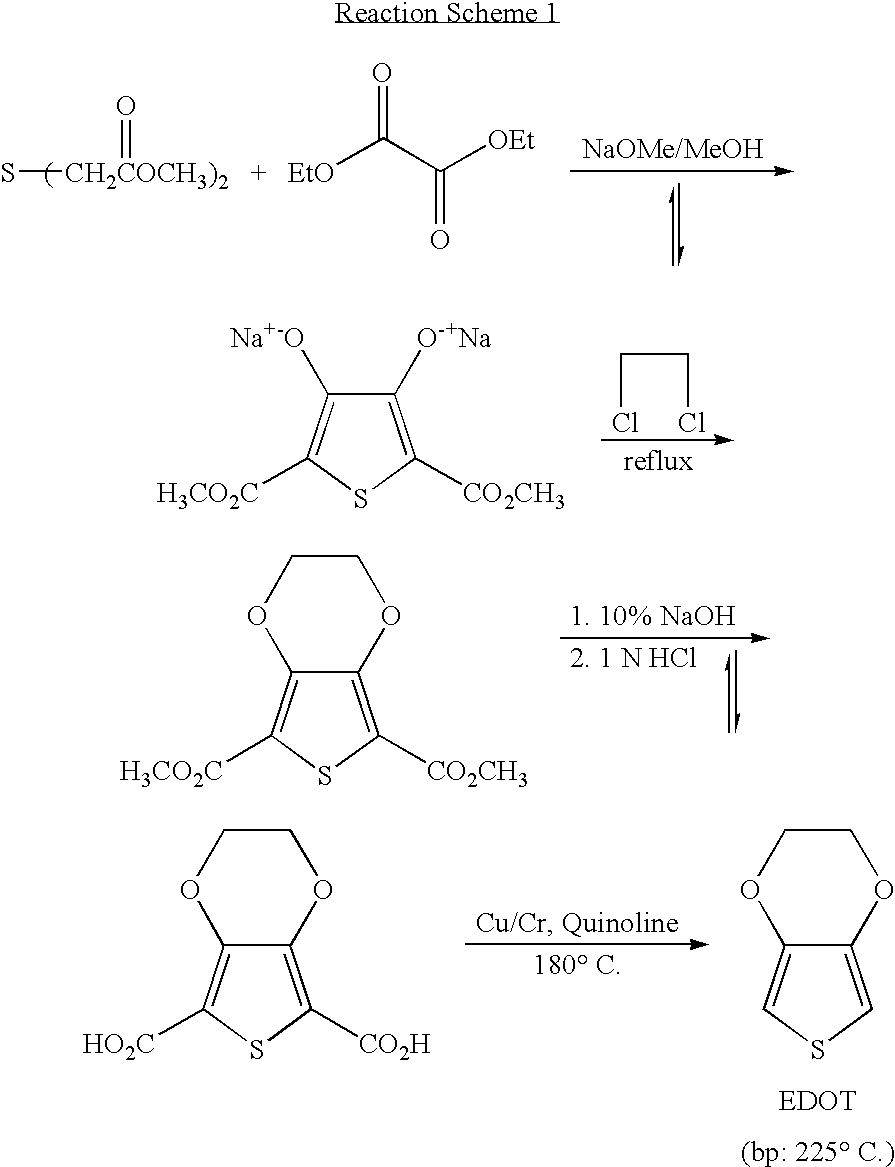
Processes for preparing of 3,4-alkylenedioxythiophenes and 3,4-dialkoxythiophenes
a technology of alkylenedioxythiophene and process, which is applied in the field of process for preparing 3, 4dialkoxythiophene and 3, 4alkylenedioxythiophene, can solve the problems of environmental pollution, impurities such as tar-like materials, and drawbacks on an industrial scale, and achieves the effect of simple and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of EDOT in DMSO in the Presence of a Copper Powder Under Oxygen
[0032]3,4-Ethylenedioxythiophene-2,5-dicarboxylic acid (460 g) and a copper powder (46 g) were added to DMSO (1200 g) at room temperature. The reaction mixture was stirred under an oxygen atmosphere for 30 minutes at room temperature and then heated at 120° C. for 6 hours. The reaction mixture was then poured into ice water (1200 mL), and the crude product was extracted with ethyl acetate. After drying over anhydrous sodium sulfate, the solvent was removed by evaporation. The residue was vacuum-distilled at 30 mmHg to give 283 g of 3,4-ethylenedioxythiophene at high purity (99.7% or higher). The purity was confirmed by gas chromatographic analysis (compared to decane as an internal standard). The chemical structure was confirmed by mass analysis and 1H-NMR.
example 2
Preparation of EDOT in Ethylene Glycol in the Presence of a Copper Powder Under Air
[0034]3,4-Ethylenedioxy-2,5-thiophenedicarboxylic acid (460 g) and a copper powder (69 g) were added to ethylene glycol (1400 g) at room temperature. The reaction mixture was stirred under air for 30 minutes at room temperature, and air was continuously introduced into the reaction mixture. The reaction mixture was then heated at 140° C. for 8 hours. After the reaction was complete, the reaction mixture was purified using the same process as described in Example 1 to obtain 3,4-ethylenedioxythiophene (281 g, purity: 98%).
example 3
Preparation of EDOT in DMF in the Presence of Basic Copper Carbonate Under Air
[0035]3,4-Ethylenedioxythiophene-2,5-dicarboxylic acid (230 g) and basic copper carbonate (23 g) were added to DMF (600 g) at room temperature. While air was slowly introduced to the reaction mixture, it as heated at 120° C. for 5 hours. After cooling, the reaction mixture was purified using the same process as described in Example 1 to obtain 3,4-ethylenedioxythiophene (136 g, purity: 97%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com