Method for treating an acute myeloid leukemia
a myeloid leukemia and acute treatment technology, applied in the field of acute treatment of acute myeloid leukemia, can solve the problems of a large disease recurrence, poor prognosis of patients harboring calm-af10 fusion, and the ineffective treatment of patients with calm-af10 fusion by standard chemotherapeutic strategies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0230]Immunodeficient mice (NRG-SGM3) were injected with the P31-Fujioka cell line. Ten days after injection, engraftment of P31 cells in mice was confirmed by flow-cytometric assessment of the human CD45 marker (data not shown). One cohort of mice was orally administered Compound 1 and an age and engraftment matched hohort was administered vehicle control. The results are illustrated in FIG. 1, which shows a survival curves for the Compound 1 vs vehicle treated animals (n=5 mice per group, *P<0.002). The results indicate that treatment with Compound 1 significantly delayed the latency of disease in mice compared to the control.
example 2
[0231]The following study was conducted in a mouse model of acute myeloid leukemia (AML) based on intravenous (IV) injection of human MOLT-4-AML cells into male non-obese diabetic (NOD) severe combined immunodeficiency (SID) mice. MOLT-4 cells are CD3 positive. The NOD-SCID mice were obtained from Jackson Laboratory, Bar Harbor Me.
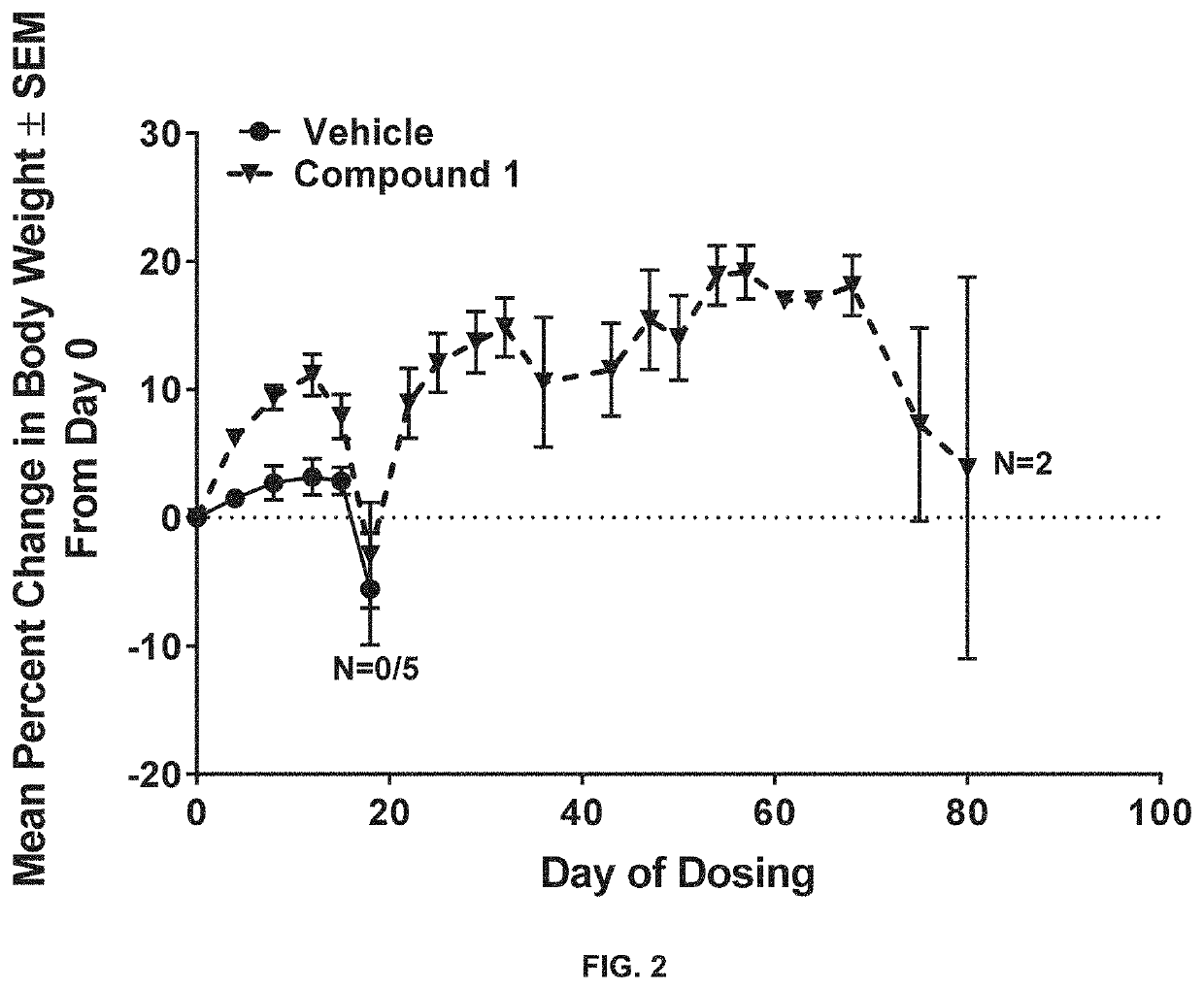
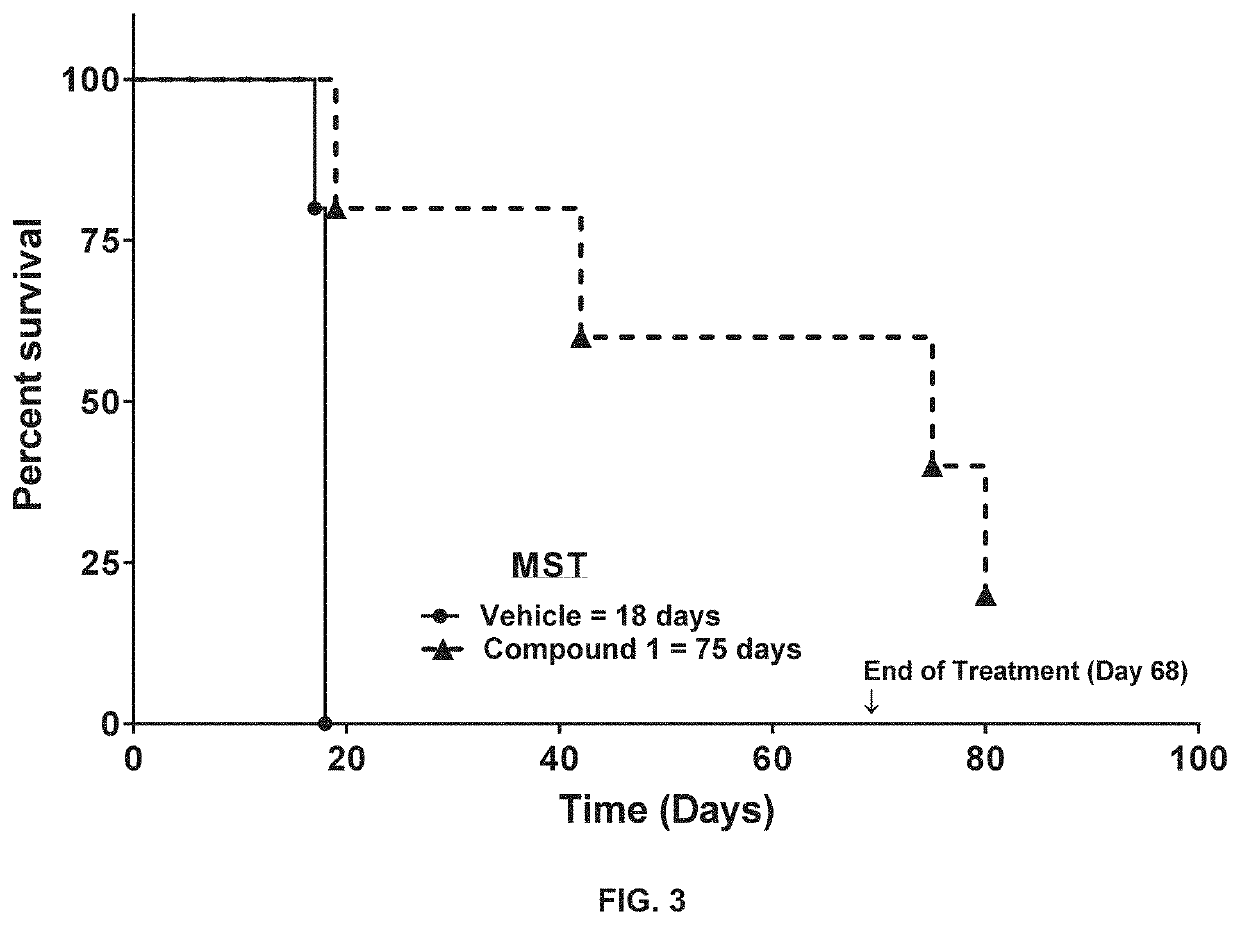
[0232]Male NOD-SCID mice were inoculated with MOLM13 human AML tumor cells from ATCC (5×106 cells in 200 μL PBS) by intravenous (IV) injection of 0.2 ml / mouse. Three days after tumor inoculation, mice were randomized into four groups of five mice per group, and each group was dosed as follows once each day: (Group 1) orally administered vehicle (0.5% HPMC), (Group 2) orally administered 12.5 mg / kg of Compound 1 in HPMC, (Group 3) orally administered 10 mg / kg of Compound 2 in HPMC, and (Group 4) orally administered a combination of 12.5 mg / kg of Compound 1 and 10 mg / kg of Compound 2 in HPMC for up to 75 days.
[0233]Results of the study are shown in FIGS. 2-5...
example 3
[0234]Male NOD-SCID mice were inoculated with MOLT human acute lymphoblastic leukemia (ALL) tumor cells from ATCC (1×107 cells in 200 μL PBS) by intravenous (IV) injection of 0.2 ml / mouse. Seven days after tumor inoculation, mice were randomized into seven groups of five mice per group, and each group was dosed as follows: (Group 1) orally administered vehicle of 0.5% HPMC and 0.1% Tween 80 daily, (Group 2) orally administered 10 mg / kg Compound 1 twice per week, (Group 3) orally administered 10 mg / kg Compound 2 daily, (Group 4) administered 1 mg / kg doxorubicin by intraperitoneal (IP) injection once per week, (Group 5) administered 0.5 mg / kg doxorubicin by IP injection once per week, (Group 6) administered 50 mg / kg Ara-C twice per week by IP injection, and (Group 7) administered 70 mg / kg Ara-C by IP injection once per week.
[0235]The resulting survival curves of the seven groups of mice from that study are shown in FIG. 6. As shown in FIG. 6, the maximum number of days of survival of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com