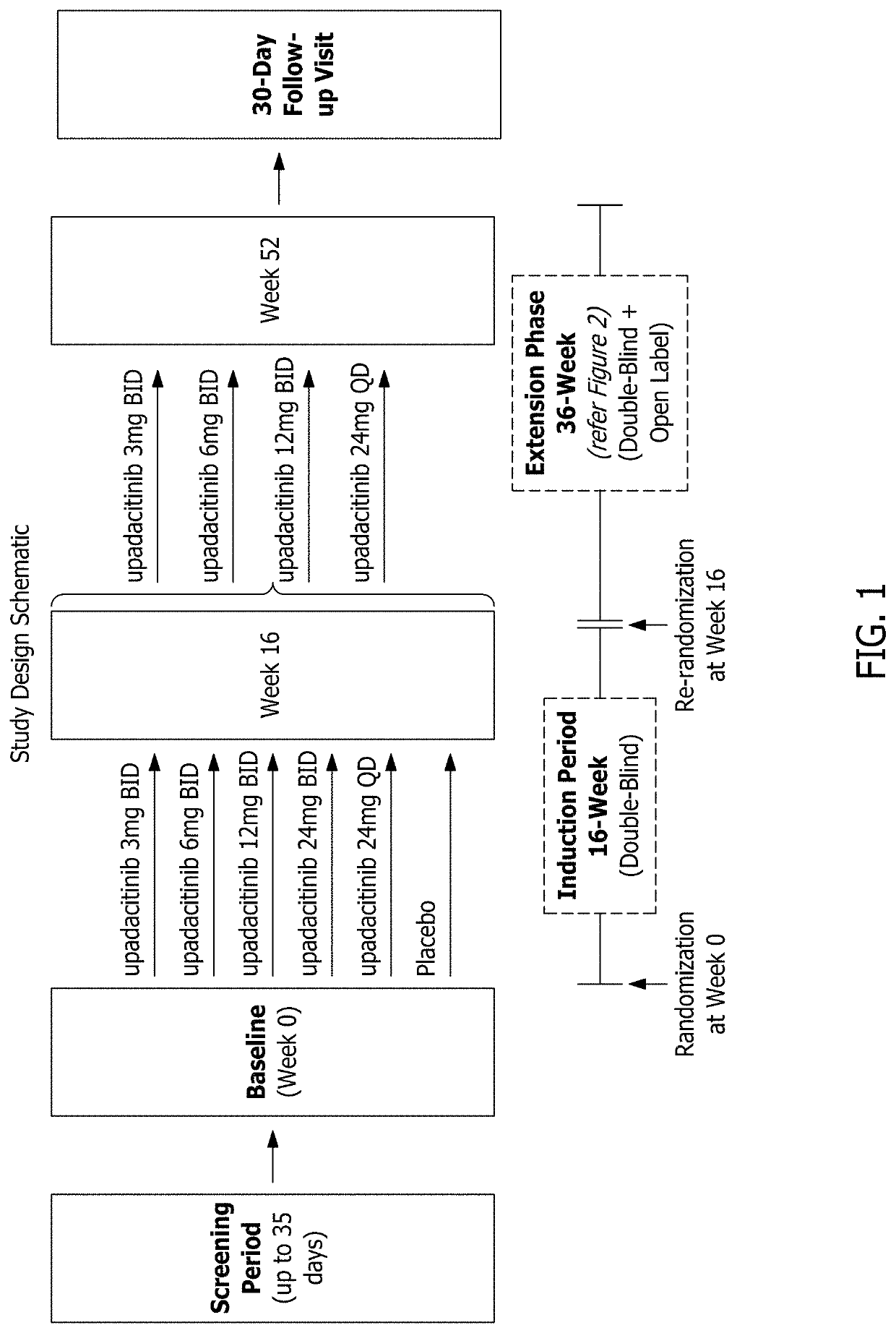
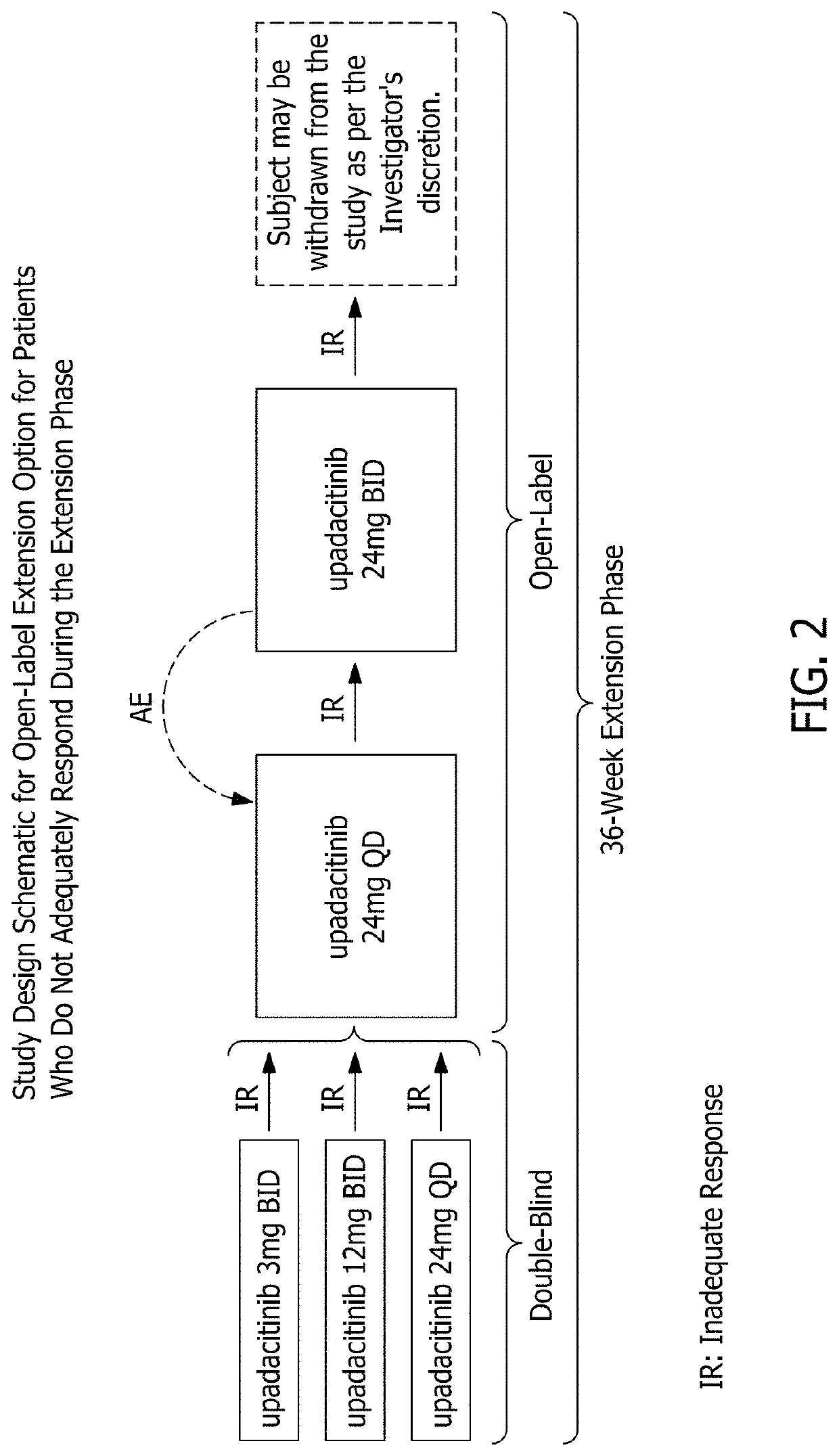
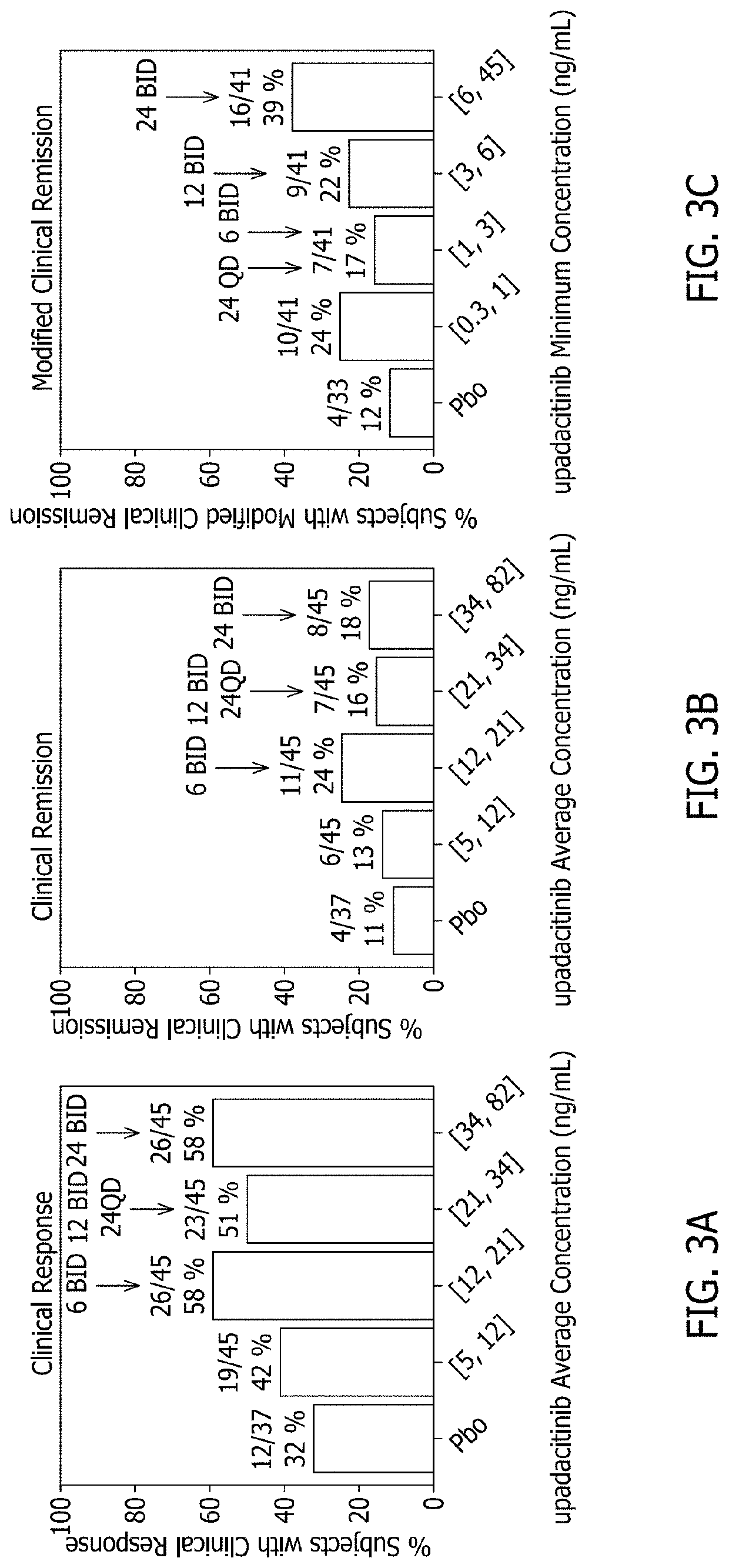
Methods of treating crohn's disease and ulcerative colitis
a technology of ulcerative colitis and crohn's disease, which is applied in the field of methods of treating crohn's disease and ulcerative colitis, can solve the problems of inability to respond to conventional treatment or experience intolerance of patients,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on of Amorphous Freebase
[0728]A. Method A: Precipitation from Water
[0729]Upadacitinib (approximately 300 g) was dissolved in water (10 L) and 50% sodium hydroxide (160 g) was added drop-wise over a two hour period to adjust the pH to greater than 12. Solids formed immediately. The solids were filtered, washed with two 500 mL aliquots of water, and then dried in a vacuum oven. The solids were equilibrated for a short period of time at ambient temperature prior to characterization. Conversion to Amorphous Freebase of upadacitinib was confirmed by PXRD analysis.
[0730]B. Method B: Dehydration of Freebase Hydrate Form B
[0731]A sample of the Freebase Hydrate Form B form of upadacitinib (crystallized from ethanol / water at sub-ambient temperatures as described in Example 2, Method C below) was placed in a vacuum oven at 40° C. overnight. The solids removed from the vacuum oven were equilibrated for a short time at 23° C. prior to characterization. Conversion to Amorphous Freebase of upadaci...
example 2
on of Freebase Solvate Form a and Freebase Hydrate Form B
[0732]A. Method A: Freebase Solvate Form a (Isopropyl Acetate / Water Solvate)
[0733]A sample of the Amorphous Freebase of upadacitinib (25 mg) was added to a vial followed by isopropyl acetate (125 μL) and water (10 μL). All solids dissolved at ambient temperature. The solution was placed in a freezer at −16° C. for 4 days. The liquor was decanted and the crystallized solids were isolated. The isolated crystals were analyzed by PXRD while still wet. Conversion to Freebase Solvate Form A (isopropyl acetate / water solvate) of upadacitinib was confirmed by PXRD analysis.
[0734]B. Method B: Freebase Hydrate Form B from Methanol / Water
[0735]A sample of the Amorphous Freebase of upadacitinib (164 mg) and MeOH (621 mg) were added to a vial. The components were mixed at ambient temperature until the solids dissolved. Water (approximately 680 μL) was added to the vial and the vial was placed in an ice / sodium chloride bath at approximately −...
example 3
on of Freebase Hydrate Form C
[0741]A. Method A: Freebase Hydrate Form C from Ethanol / Water
[0742]A sample of the Amorphous Freebase of upadacitinib (2 g) was transferred to a 500 mL beaker equipped with a stirring bar. EtOH (50 g) was added to the beaker and stirred until all solids dissolved. The solution was transferred to a 250 mL jacketed flask equipped with a dispersing device. The solution was cooled to 6° C. Water (150 g) was added to the solution and the solution was subjected to high shear for two hours using the dispersing device. After solid formation was observed, an additional amount of water (50 g) was added to the resulting suspension. The suspension was held overnight at ambient temperature. Solids were isolated and examined on the following day. Conversion to upadacitinib Freebase Hydrate Form C was confirmed by PXRD analysis.
[0743]B. Method B: Freebase Hydrate Form C from Ethyl Acetate / Heptane / Water
[0744]A crude reaction mixture assaying for 11.1 g of upadacitinib w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com