Therapeutic uses of fibrinogen gamma prime variants
a fibrinogen and prime variant technology, applied in the field of fibrinogen variants comprising a prime chain, can solve the problem that it is not likely that the amount of fibrinogen variants is sufficien
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
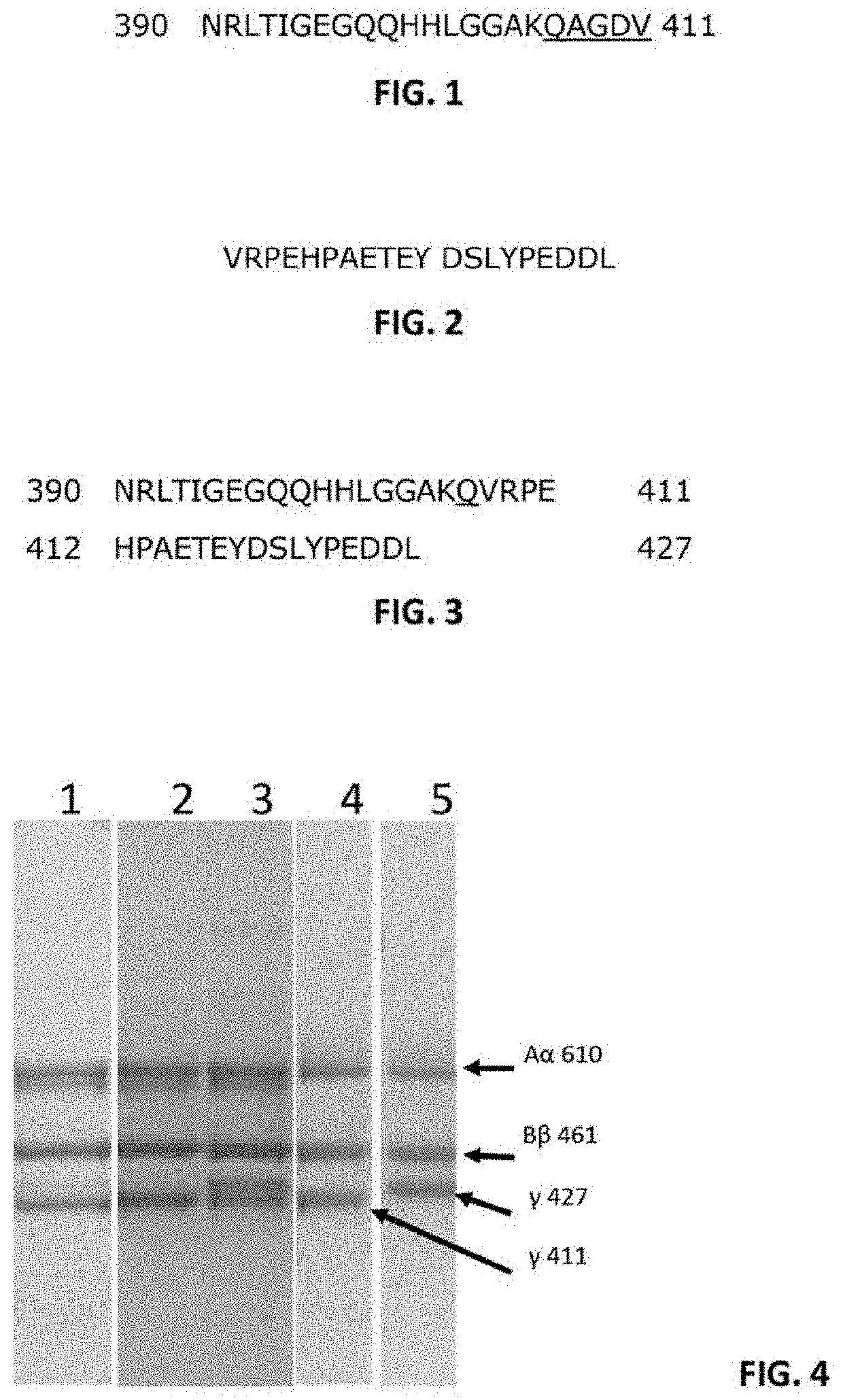
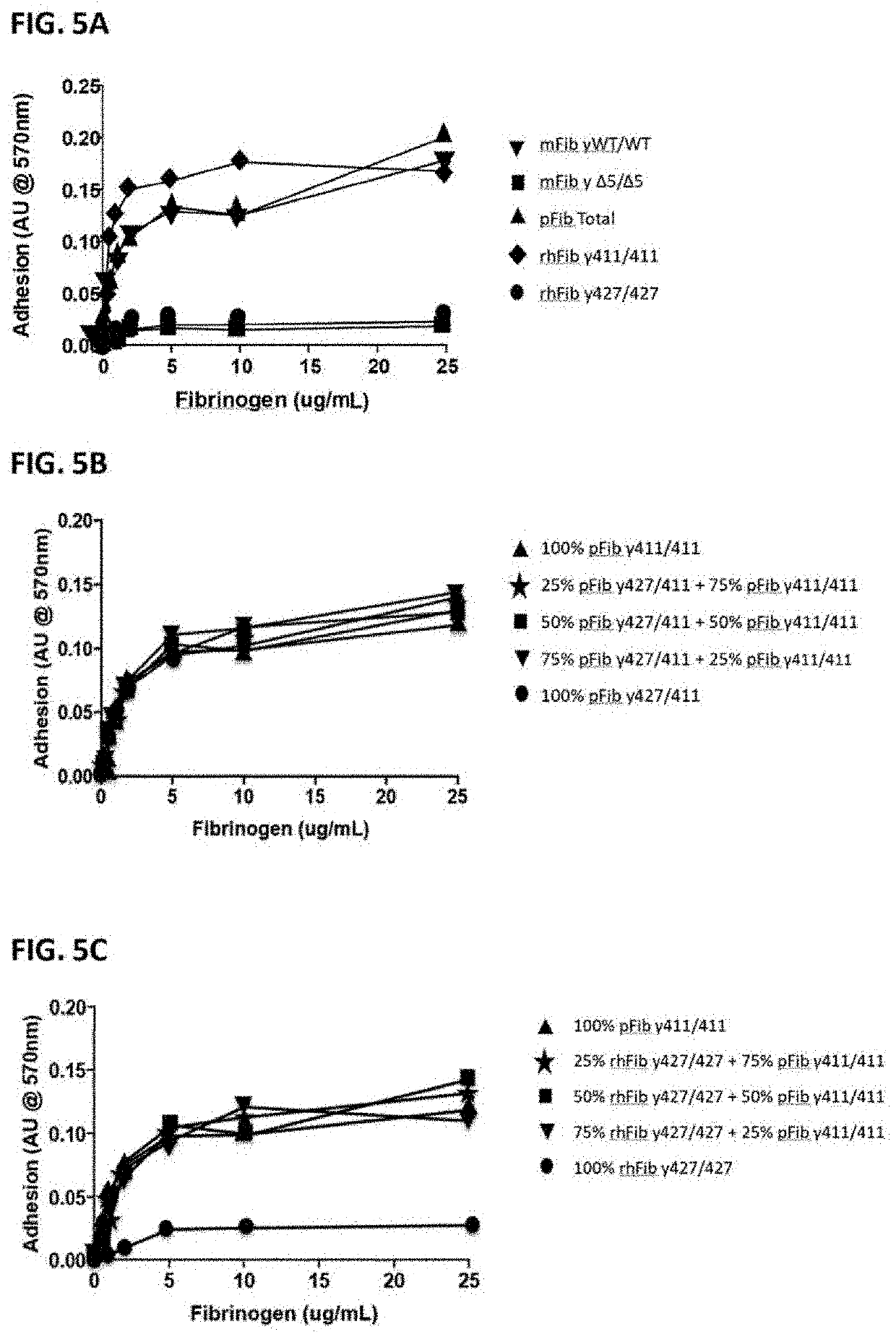
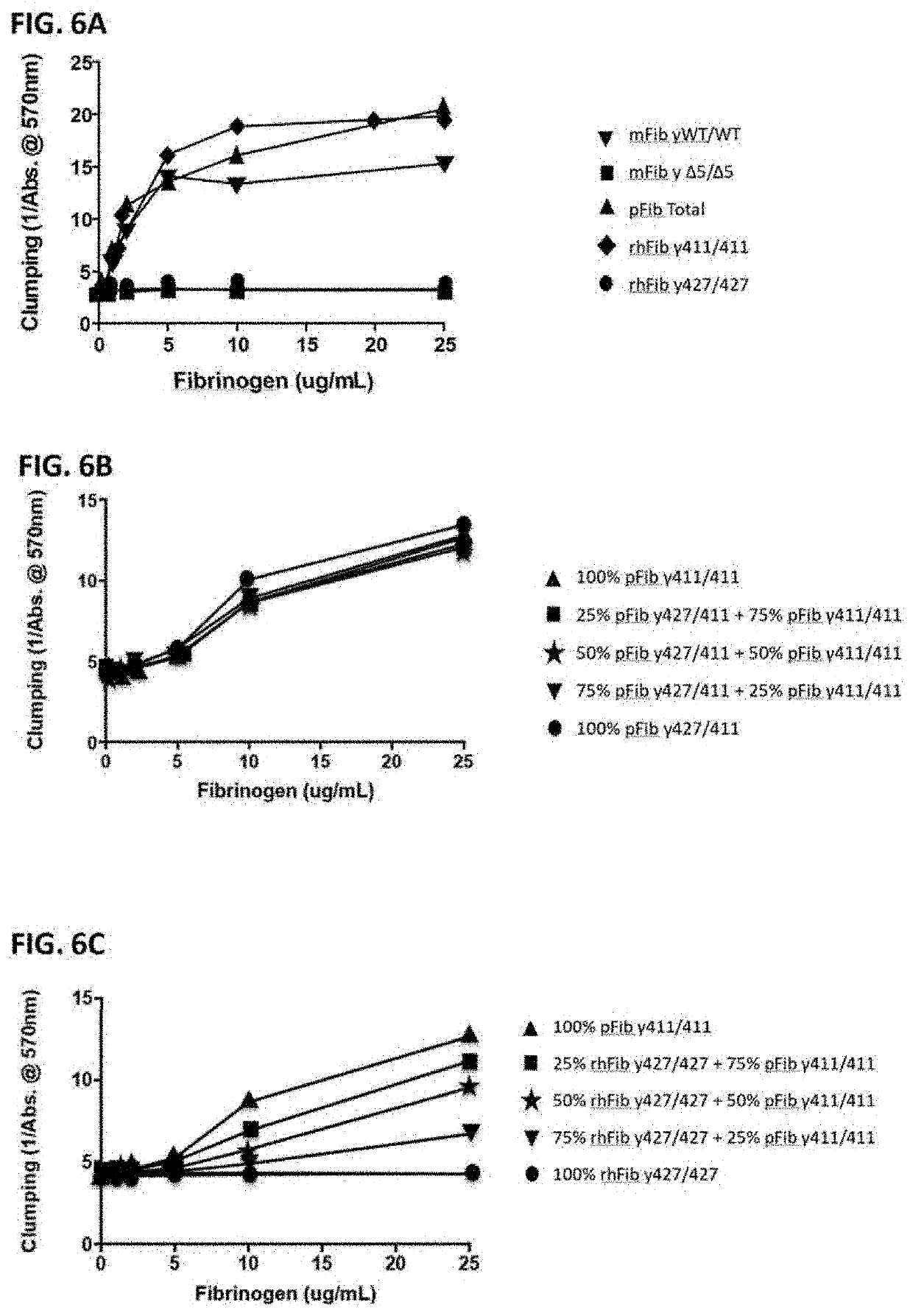
Isolation of Fibrinogen Variants from Plasma
[0093]Total plasma fibrinogen mixture (pFib total) comprising wild type (WT) fibrinogen (pFib γ411 / 411) and fibrinogen gamma prime (pFibγ427 / 411) was obtained from Enzyme Research Labs, Swansea, United Kingdom (FIB 3). This was used as starting material to separate the plasma fibrinogen with two WT gamma polypeptide chains (pFib γ411 / 411) from the plasma fibrinogen variant with one gamma prime polypeptide chain and one WT gamma polypeptide chain (pFib γ427 / 411) using anion exchange chromatography as described in Lawrence et al. (Blood 1993, vol 82, no 8, pp 2406-2413). pFib total, pFib γ411 / 411 and pFib γ427 / 411 were analysed on SDS-PAGE (Example 3) and used in further experiments as described below.
[0094]Mouse fibrinogen from WT mice (mFib γWT / WT) and from homozygous delta5 transgenic mice (mFib γΔ5 / Δ5) was isolated as described by Flick et al. (2013) Blood (121): 1783-1794.
example 2
Recombinant Production of WT Fibrinogen and Homodimer Fibrinogen Gamma Prime
[0095]cDNA sequences encoding the fibrinogen Aα610 chain (Alpha wild type, SEQ ID NO. 4), Bβ chain (SEQ ID NO. 5), 7411 (gamma) chain (SEQ ID NO. 6) and γ427 (gamma prime) chain (SEQ ID NO. 7) were cloned in the pCDNA 3.1 plasmid (Invitrogen, Carlsbad, Calif., USA) to construct expression vectors for the different fibrinogen chains. Combinations of expression plasmids containing cDNA's encoding Aα, Bβ and γ chains were used to produce fully assembled recombinant human WT fibrinogen (rhFib γ411 / 411) and recombinant fibrinogen gamma prime homodimer (rhFib γ427 / 427) by transient expression in HEK 293 cells (Life technologies EXPI293 system) according to the manufacturer's instructions. These recombinant fibrinogen variants were purified using affinity purification on a GPRP column as described in Kuyas C et al. (Thrombosis Haemostasis 1990 Jun. 28; 63 (3): 439-444) and analysed on SDS-PAGE (as described in Exam...
example 3
SDS-PAGE Analysis of Fibrinogen Isolated from Plasma and Recombinantly Produced Fibrinogen
[0096]pFib γ411 / 411 and pFib γ427 / 411 isolated from total plasma fibrinogen (pFib total) were subjected to SDS-PAGE analysis under reducing conditions. About 0.5 to 1.0 microgram of the fibrinogen samples was loaded on the SDS-PAGE gel, run for 1 hour at 200 Volt and stained with Coomassie blue stain. The result is shown in FIG. 4.
[0097]Lane 1: pFib total (total plasma fibrinogen) showed as dominant bands the Aα610, Bβ 461 and γ411. A slightly degraded Aαband is seen just below the Aα610 band and a faint band at the γ427 position is present. Lane 2: pFib γ411 / 411 only contains only the γ411 band at the gamma polypeptide position. Lane 3: pFib γ427 / 411 isolated from pFib total contains wild type Aα610 and Bβ461 polypeptides, but the γ chain polypeptide consists of approximately equal amounts of γ411 and γ427, demonstrating that pFib γ427 / 411 is a heterodimer with respect to the gamma polypeptide...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com