Method for thermo-chemical energy storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
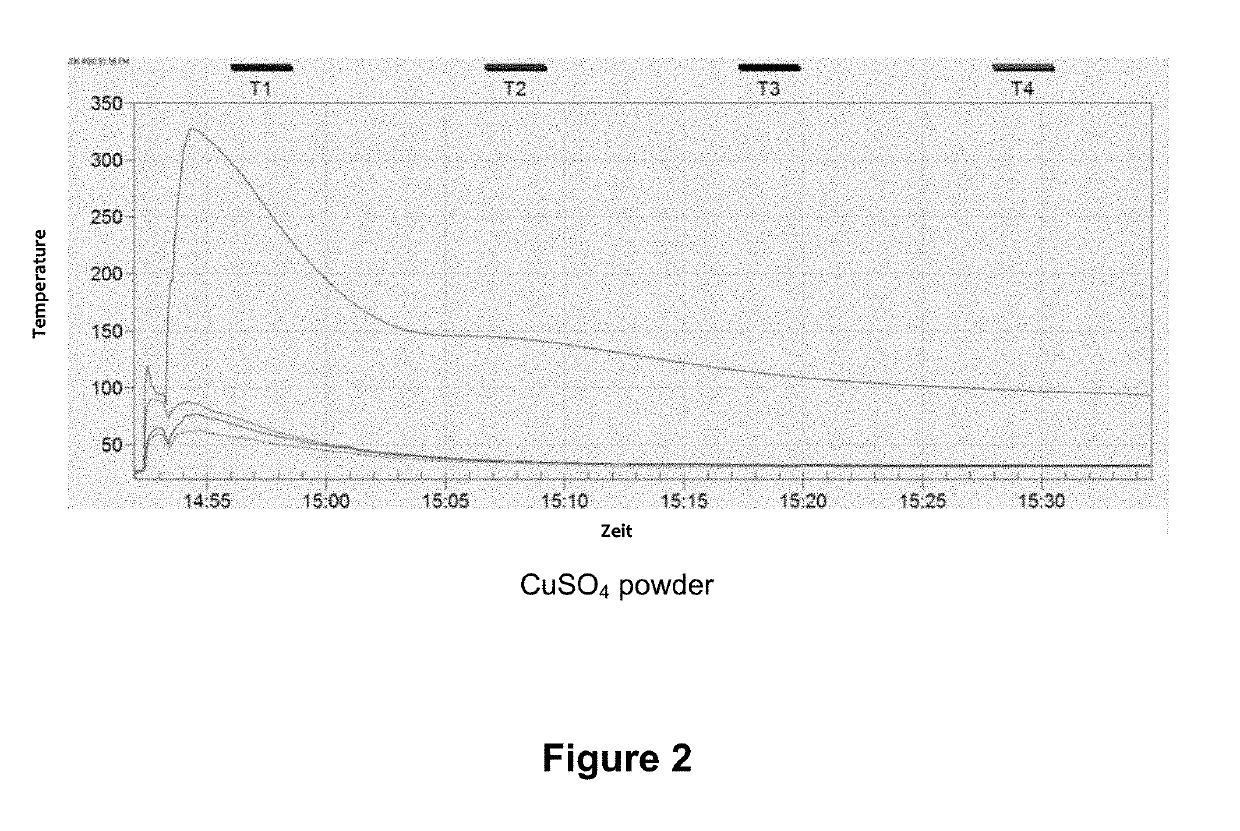
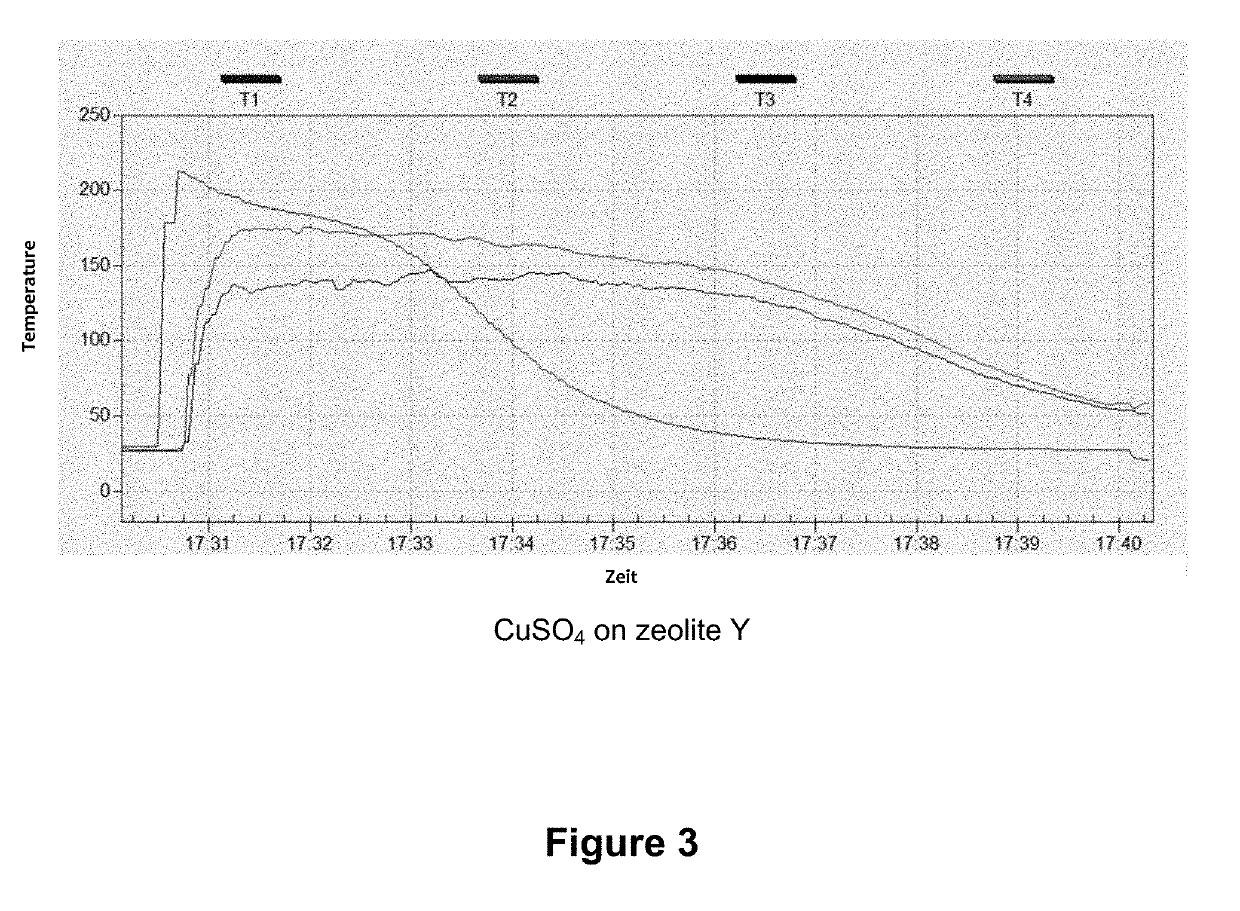
[0031]In a typical loading experiment, 50 g of zeolite Y, which had previously been dried for 6 h at 400° C., were immersed into a saturated CuSO4 solution for 2 h and thus soaked therewith. The bluish product was then separated, flushed with 200 ml of deionized water, and dried for 12 h at 60° C. and thereafter for 6 h at 350° C. A CuSO4 content of 39.7 wt % was determined using XRF analysis.
synthesis example 2
[0032]In a manner analogous to Synthesis Example 1, zeolite Y was soaked with a saturated CuCl2 solution. A CuCl2 content of 44.3 wt % was determined using XRF analysis.
synthesis example 3
[0033]In a manner analogous to Synthesis Example 1, zeolite Y was soaked with a saturated CdCl2 solution. A CdCl2 content of 59.5 wt % was determined using XRF analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com