Analogs of cyp-eicosanoids for use in treating or preventing a disorder associated with neovascularization and/or inflammation
a technology of cypeicosanoids and epoxymetabolites, which is applied in the field of analogues of cypeicosanoids for use, can solve the problems of not being used as therapeutics, prone to autoxidation of epoxymetabolites of n-3 pufas, and the half-life of said compound may be longer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Efficacy of Compound 1 in a Laser Induced Choroidal Neovascularization Model in Rat Evaluated by Measuring Vascular Leakage Using Fluorescence Angiography
[0499]The laser induced CNV model in rat is a widely used model to prove the therapeutic efficacy of drugs for treatment of several ocular diseases characterized by angiogenesis and inflammation in the eye such as wet age-related macular degeneration (AMD). In this model a focused laser burn in the layer of Bruch's membrane causes a disruption of this membrane, a local injury, followed by inflammation and growth of new blood vessels. These newly formed blood vessels are typically leaky. This leakage is measured by extravasation of a fluorescent dye in the back of the eye (Fluorescein) and is a well-accepted marker for vascular leakage which is proportionally to the amount of newly grown blood vessels. This method is also the state of the art procedure in the clinical practice in man.
[0500]All standard operating procedures and proto...
example 3
Comp-02 Distribution after Oral, Topical or Intravitreal Administration in Different Compartments of the Rabbit Eye
[0508]This example shows that highest ocular exposures of Comp-02 in the posterior eye are reached after intravitreal injection.
Materials and Methods
[0509]Study design: To gain insight into the distribution behaviour of synthetic 17,18-EEQ-agonists, ocular pharmacokinetic studies were performed in pigmented HY79b rabbits. Briefly, 15 male rabbits per administration route were anesthetized at the time-points for sampling indicated in tables 2-4 by an intramuscular injection of a mixed solution of xylazine and ketamine. Blood collection was performed by cardiac puncture in K3EDTA tubes and centrifuged at 2000 g, 10 min at 4° C. Approximately 3 mL of plasma were sampled, put in plastic tubes, snap frozen in liquid nitrogen and stored at −80° C. until assay. Then animals were euthanized by intracardiac injection of overdosed pentobarbital. This method is one of the recommen...
example 4
Anti-Inflammatory Effect of a Metabolically Robust Analog of 17,18-EEQ (Comp-02) on HL-1 Cardiomyocytes
Materials and Methods
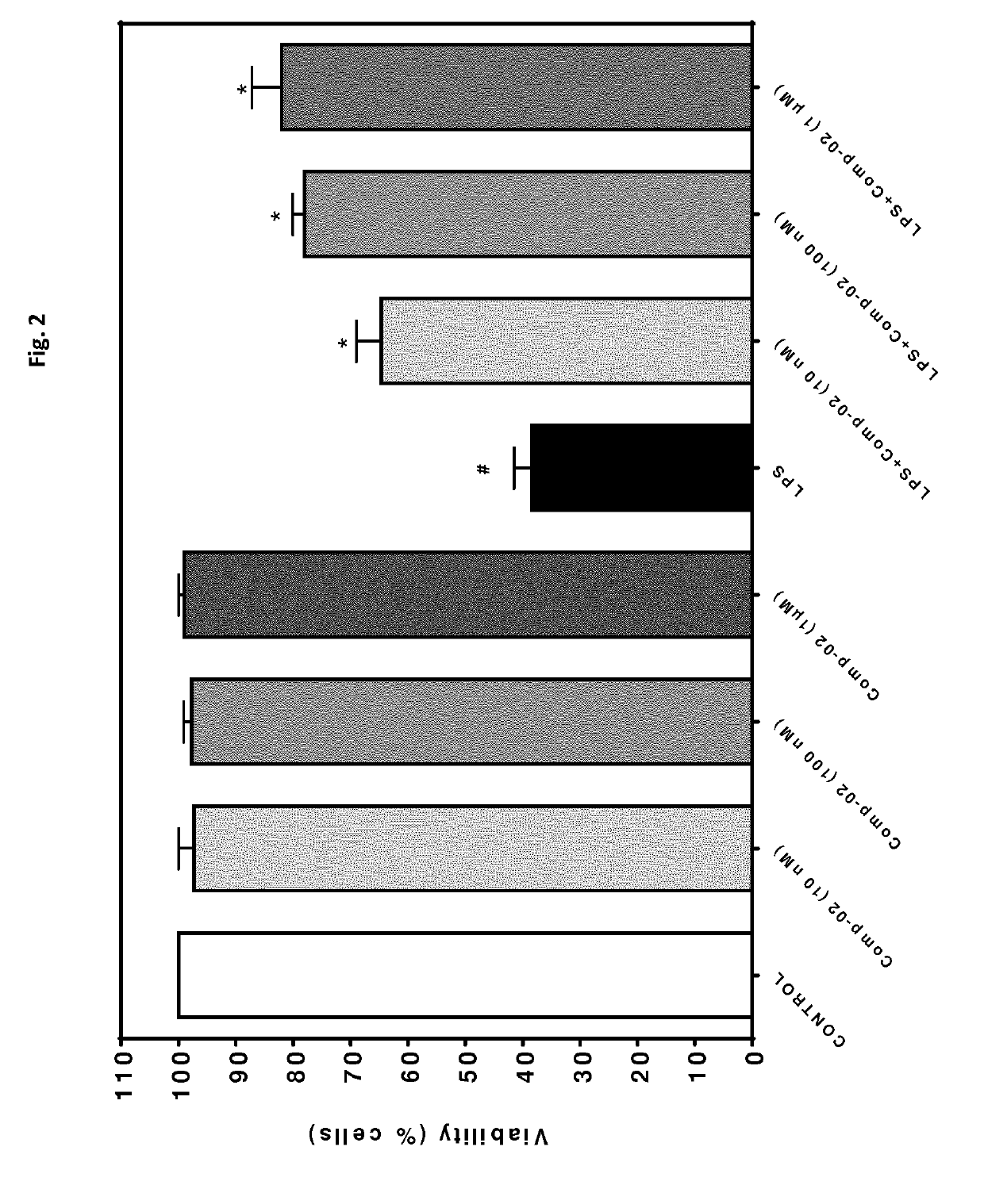
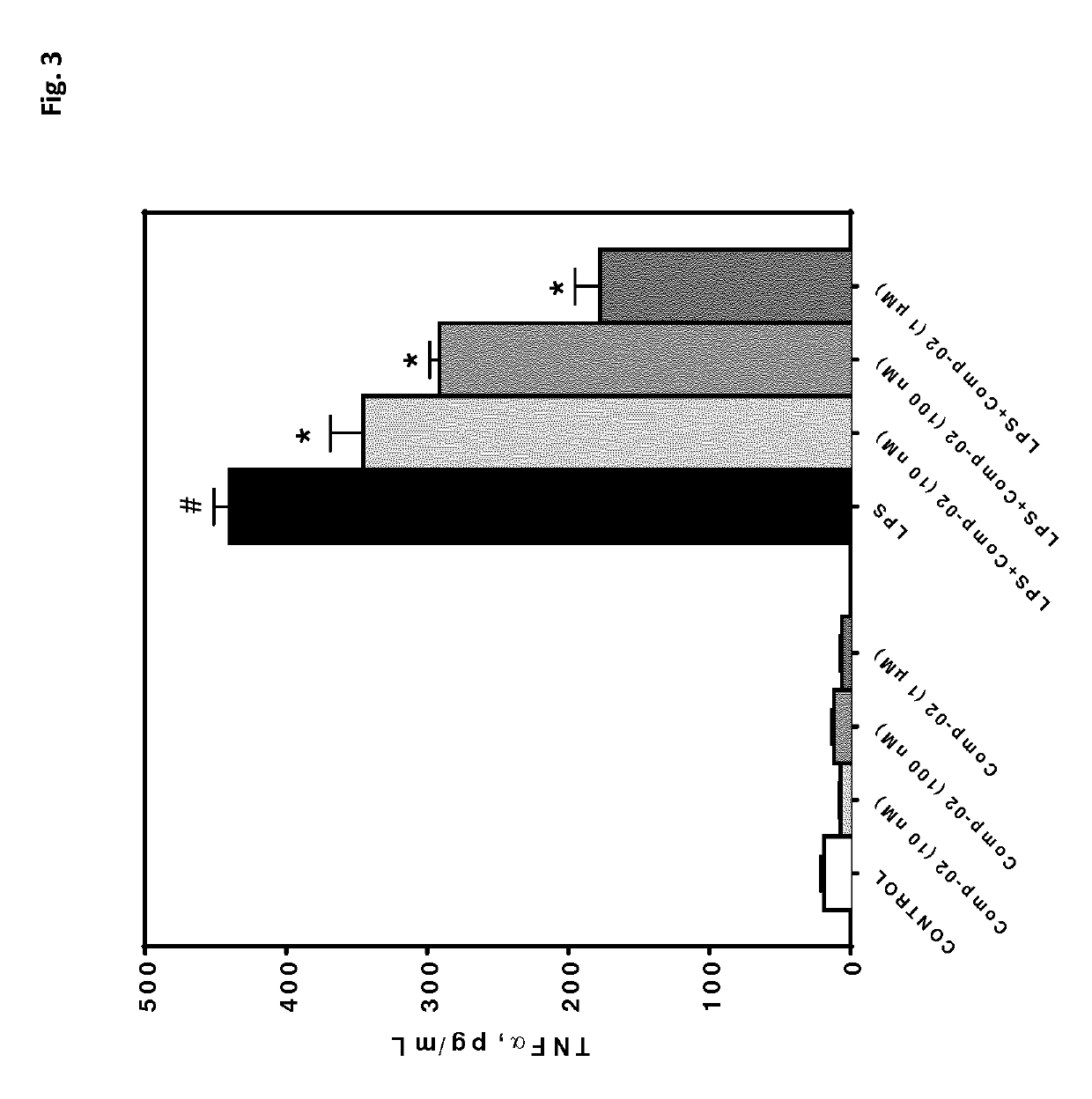
[0514]In order to investigate the anti-inflammatory potential of compounds being part of the invention in vitro, a cardiomyocyte cell line was used (mouse derived immortalized cardiomyocytes, HL-1 cells). Cells were either treated with vehicle (0.01% ethanol) or different concentrations of test compound (Comp-02: cE=10 nM, 100 nM or 1 μM). Simultaneously, the cells were challenged with 1 μg / mL lipopolysaccharide (LPS). After 24 h of incubation, the cells were processed to measure viability (FIG. 2) and release of the pro-inflammatory cytokine TNF alpha (FIG. 3).
Results
[0515]The results are presented in FIG. 2 and FIG. 3. The inflammatory stimulus LPS leads to a significant reduction in cell viability. This cytotoxic effect was dose-dependently reversed by Comp-02, FIG. 2. Moreover, LPS-incubation significantly induced the production of the pro-inflammatory cyto...
PUM
| Property | Measurement | Unit |
|---|---|---|
| vascular permeability | aaaaa | aaaaa |
| electrical | aaaaa | aaaaa |
| L-type Ca2+ currents | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com