Site-specific nuclease single-cell assay targeting gene regulatory elements to silence gene expression
a gene regulatory element and single-cell technology, applied in the direction of hydrolases, microorganism testing/measurement, biochemistry apparatus and processes, etc., can solve the problem that current sirna and 3c-based experimental approaches cannot be applied to multigene complexes, cannot reveal the necessity of chromosomal contacts for cotranscription, and cannot eliminate the transcriptional activity of the gene of interest. , to achieve the effect of reducing the capacity of a gene loop, o
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0132]Cell Culture
[0133]Early passage HUVECs from pooled donors (Lonza) were grown to ˜80% confluence in Endothelial Basal Medium-2 (EGM-2) with supplements (Lonza), serum-starved (18 hr) in EGM-2+0.5% FBS, and treated with TNFα (10 ng / ml; Sigma) for up to 30 mins. Prior to transfection cells were grown in antibiotic free EGM-2.
[0134]TALEN Design
[0135]Software developed by the Bogdanove laboratory was used to identify TALEN candidate binding sites (Doyle et al., 2012). Left and right TALENs were designed to contain 18 full monomer repeats, which target a 20 bp sequence, where the first and last bases are specified by the thymine at the N terminus, and the 0.5 repeat, respectively. To facilitate FokI dimerization, the left and right TALEN target sites were chosen with a spacer of 16-19 bp. For the pDT TALEN vector, the SAMD4A left and right arms were cloned into the pBI_CMV1 bidirectional promoter vector (Clontech). The left TALEN was cloned into MCS1 of pBI_CMV1 (MluI and HindIII) a...
example 2
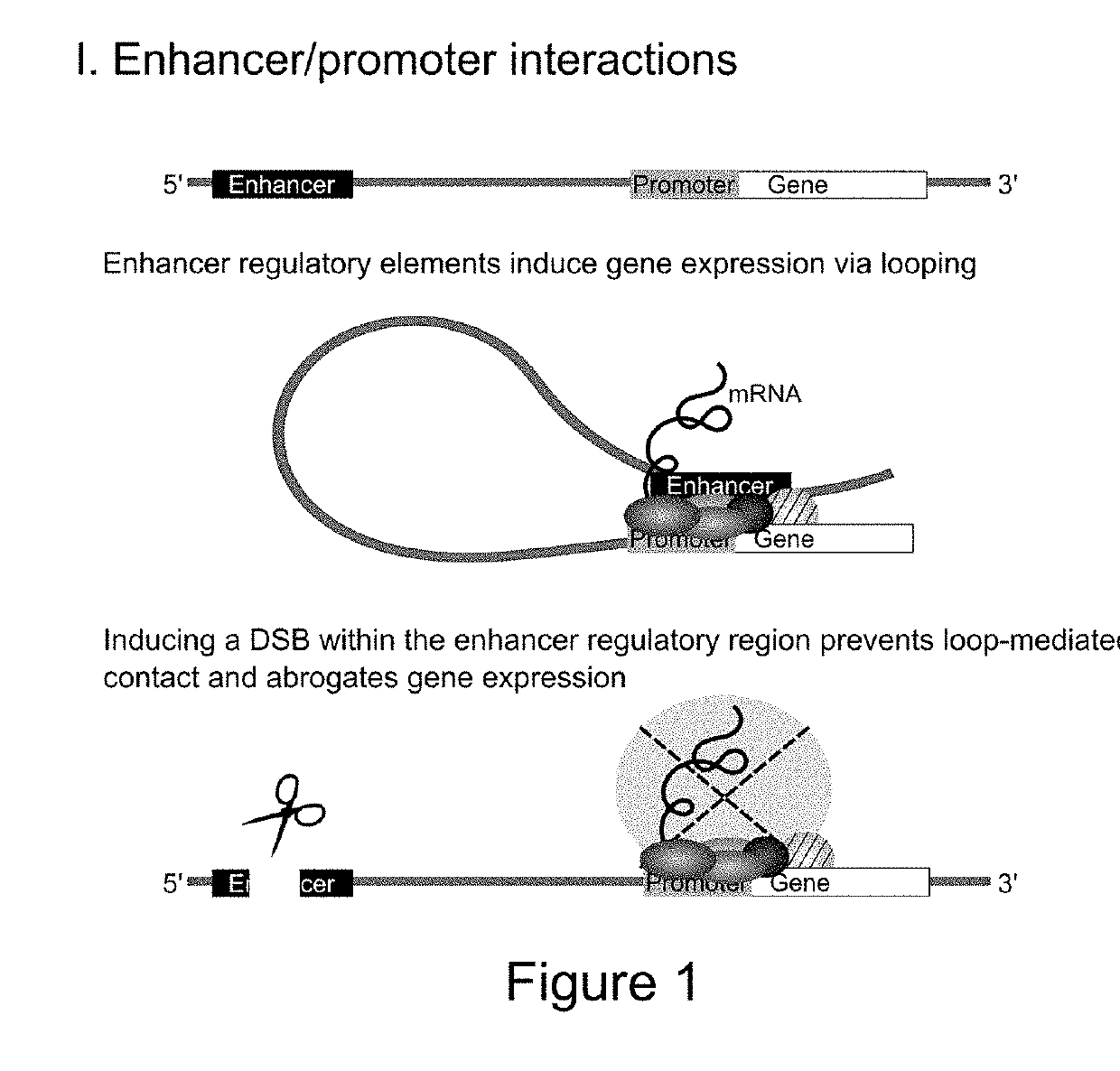
[0174]Disrupting the IL8 Enhancer Abrogates IL8 Expression
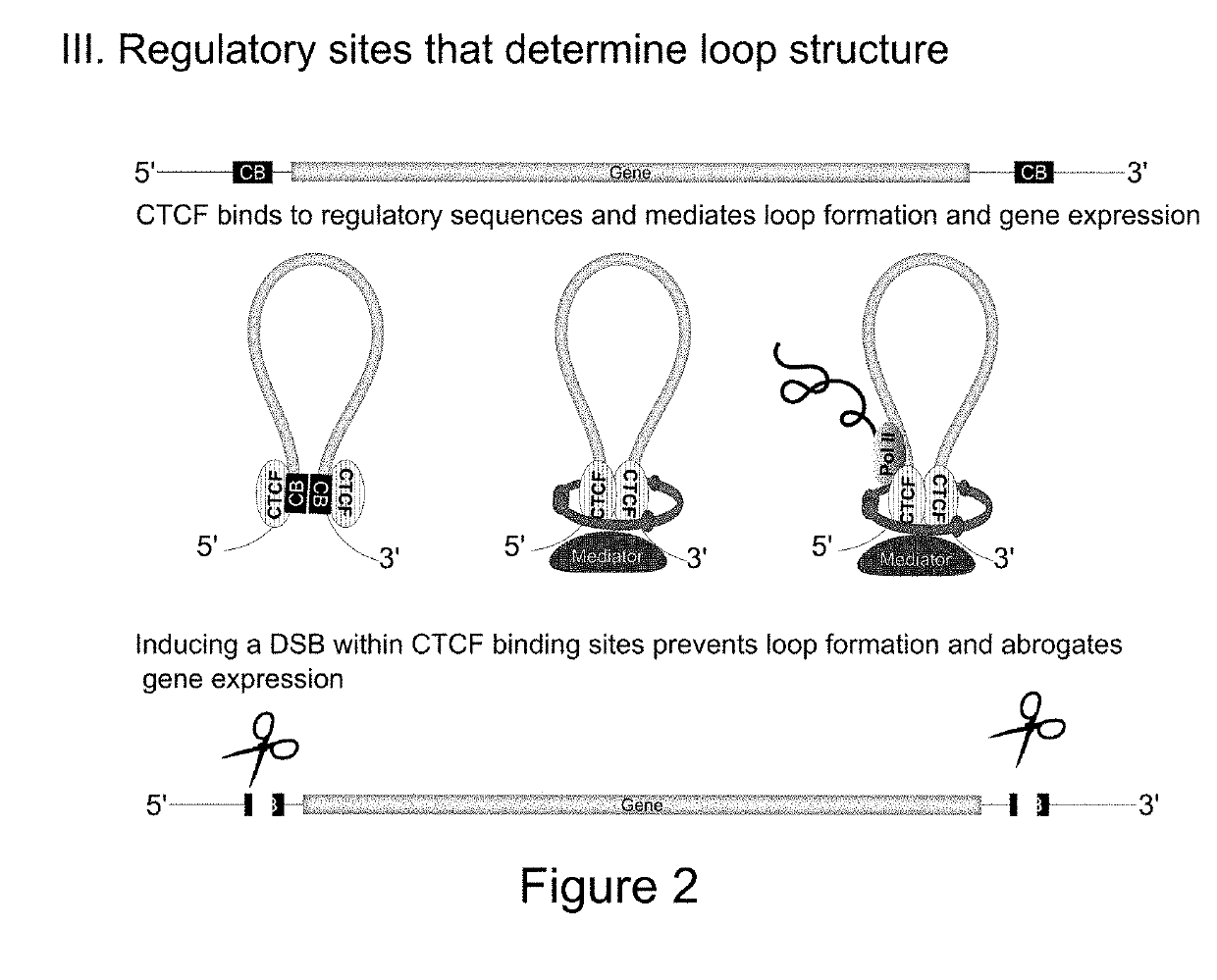
[0175]Recently, Hi-C studies revealed that the pro-inflammatory chemokines CC Chr.17 (CCL2, CCL7, CCL11) and CXC Chr.4 (IL8, CXCL1, CXCL3, CXCL2) are organized into TADs and engage in chromosomal contact.
[0176]Using intronic smFISH, we were able to show that the CXC chemokines are only induced following TNF induction. Furthermore, the smFISH foci of co-expressed CXC genes always co-localize.
[0177]Deeper bioinformatic analysis of both Hi-C and ChIA-PET data in the CC and CXC TADs identified a large cluster of transcriptional enhancers. At the 5′ end of the CXC TAD, we identified a putative ‘super-enhancer’ region, spanning ˜80 kb and forming extensive chromosomal contacts with the proinflammatory genes (unpublished data). Typically, such regions are densely occupied by chromatin regulators over tens of kb. Accordingly, this region is highly enriched for eRNA chromatin marks, H3K4me1 and H3K27Ac. Using the recently published ‘e...
example 3
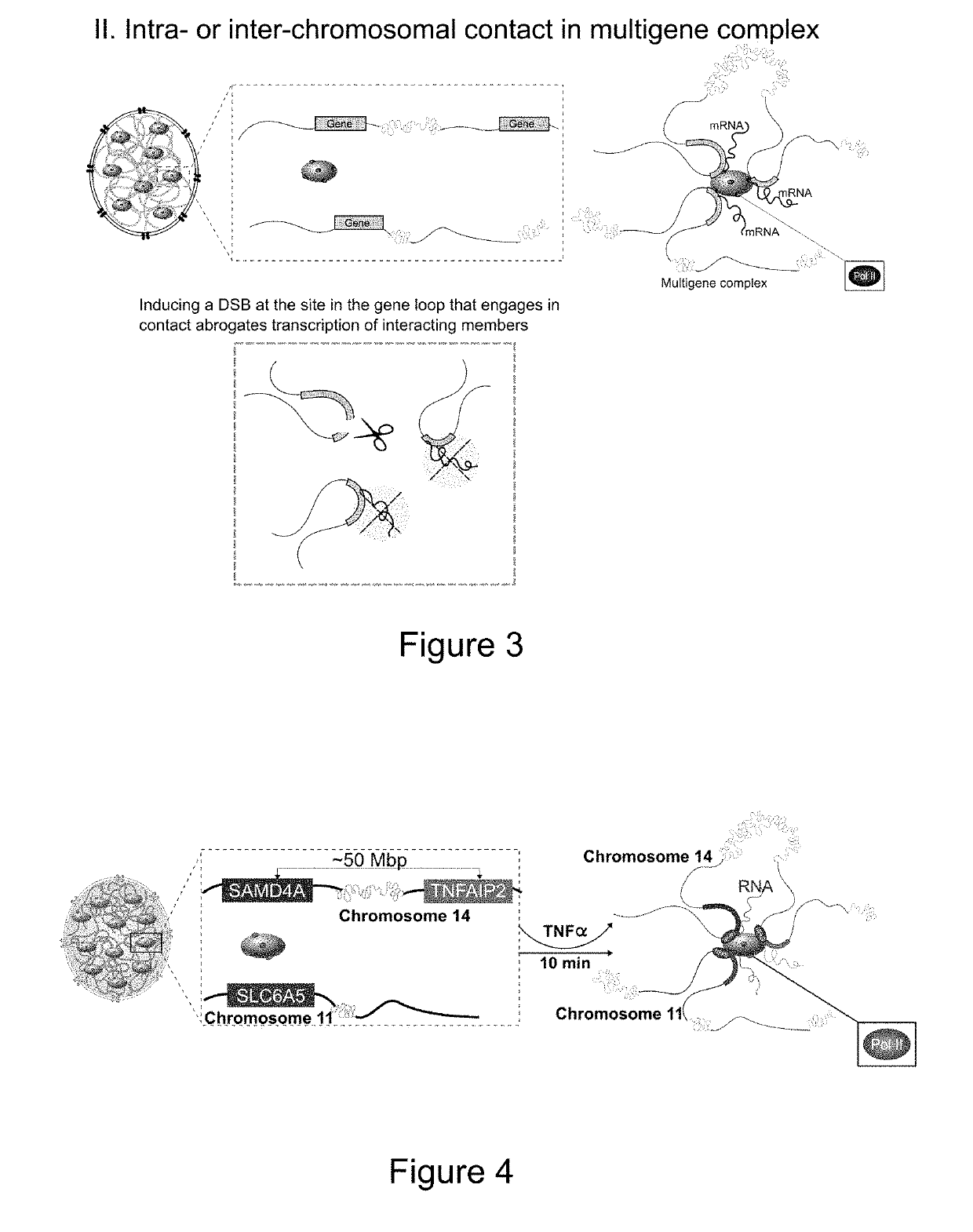
[0179]Disrupting Intra- or Interchromosomal Contact in Multigene Complexes Abrogates the Transcription of Interacting Genes (See FIG. 52)
[0180]TNFα, a major proinflammatory cytokine, is a stimulus that induces the coordinated assembly of coregulated genes in NF-κB-dependent multigene complexes (Papantonis et al., 2012). SAMD4A, a ˜221 Kb gene on chromosome 14, is rapidly switched on by TNFα in primary human umbilical vein endothelial cells (HUVEC). 4C analysis reveals that prior to stimulation with TNFα, SAMD4A seldom interacts with other genes.
[0181]After activation by TNFα, SAMD4A interacts with multiple coregulated genes to form a multigene complex. TNFAIP2, a gene located on the same chromosome but ˜50 Mb downstream, and SLC6A5 on chromosome 11, are two well-characterized interacting partners of SAMD4A. siRNA approaches cannot be utilized to interrogate loop-mediated dynamics in the SAMD4A / TNFAIP2 / SLC6A5 multigene complex, as all three genes are activated by the same transcripti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| exposure time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com