Therapeutics for preterm labor management
a technology for preterm labor and treatment, applied in the field of biological and medical science, can solve the problems of perinatal morbidity and mortality affecting 12, premature newborns are at increased risk for acute and chronic health problems, developmental deficiencies, marginal efficacy and potential adverse effects on the fetus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Liposome (lip) Prevents the Transfer of Indomethacin Across the Placenta to the Fetus
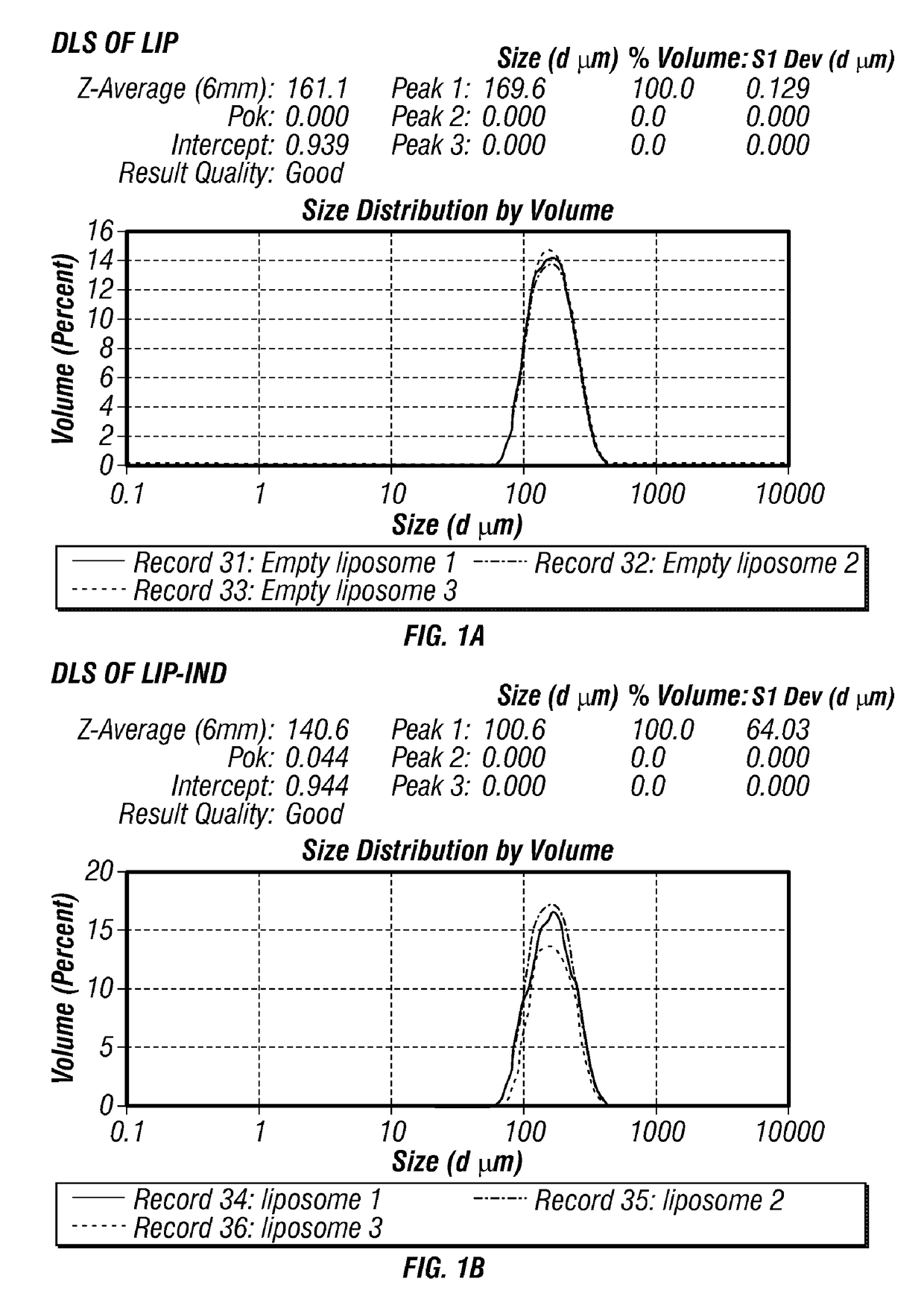
[0051]To determine whether liposomes (LIP) could prevent the transfer of indomethacin (IND) across the placenta to the fetus while preserving its pharmacological activity, multi-lamellar LIP were designed with a 150-200 nm size, fluorescently labeled and loaded with IND (LIP-IND). Characterizations of the liposomes by DLS and SEM showed that the nanoparticles appear as uniform, spherical vesicles of ˜150-170 diameter (FIG. 1). When the size distribution of five separately prepared batches of IND and LIP-IND was evaluated, the average values were 159.8±1.1 nm (polydispersity index, PDI<0.083) for LIP and 164.4±4.7 (PDI: 0.069) for LIP-IND. The low PDI (<0.1) values point towards the homogeneity of the formed phospholipid nanovesicles. Quantitative analysis of the drug revealed that IND encapsulation efficiency in the liposomes was 93%. To enable the biodistribution in the tissues analysis, LIP and IN...
example 2
Targeted Delivery of LIP-IND to the Pregnant Uterus
[0056]Liposome design and fabrication. To achieve active targeting of the LIP-IND system to the uterus, a new method was developed, which involved conjugating clinically used ORA to the liposome's surface. Liposomes loaded with IND and decorated with a clinically available oxytocin receptor antagonist (Flenady et al., 2014) (ORA) on its surface were fabricated into LIP-IND-ORA, as schematically presented in FIG. 5. Oxytocin receptors are specifically expressed on the pregnant uterus. Accordingly, the LIP-IND-ORA were evaluated to determine their ability to specifically direct the delivery of IND to the pregnant uterus, inhibit uterine contractions, and reduce preterm birth.
[0057]For this purpose, the liposomes were engineered to include phospholipids with a spacer and carboxylic group (PEG-DSPE), which can react with the amino group of the ORA using a post-insertion technique. Various concentrations of the constituents were tested. ...
example 3
Materials and Methods
[0067]Liposome design and fabrication. LIP, LIP-IND, LIP-ORA and LIP-IND-ORA were prepared by lipid hydration-extrusion method. First, the lipids were dissolved in 3 mL ethanol at the following concentrations: 9.6-12.2 mg soy bean phosphatidylcholine (Lipoid 5100, Lipoid, Germany), 0-0.77 mg cholesterol (Sigma) and 1-3 mg DSPE-PEG(2000) Carboxylic Acid (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[carboxy(polyethylene glycol)-2000] (ammonium salt)) (Avanti, Alabama, USA). To fluorescently label LIP, fluorescent phospholipid Lissamine rhodamine B 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt (rhodamine-DHPE, Invitrogen), 2% of the total lipid was incorporated to all liposome formulations. 0.45 mg of IND (Sigma) was added to the above ethanolic mixture for LIP-IND and LIP-IND-ORA formulations. A thin film was formed by evaporating the solvent for 30 minutes (min), 41° C. at 150 rpm using rotary evaporator (Rotavapor, Buchi, Switz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com