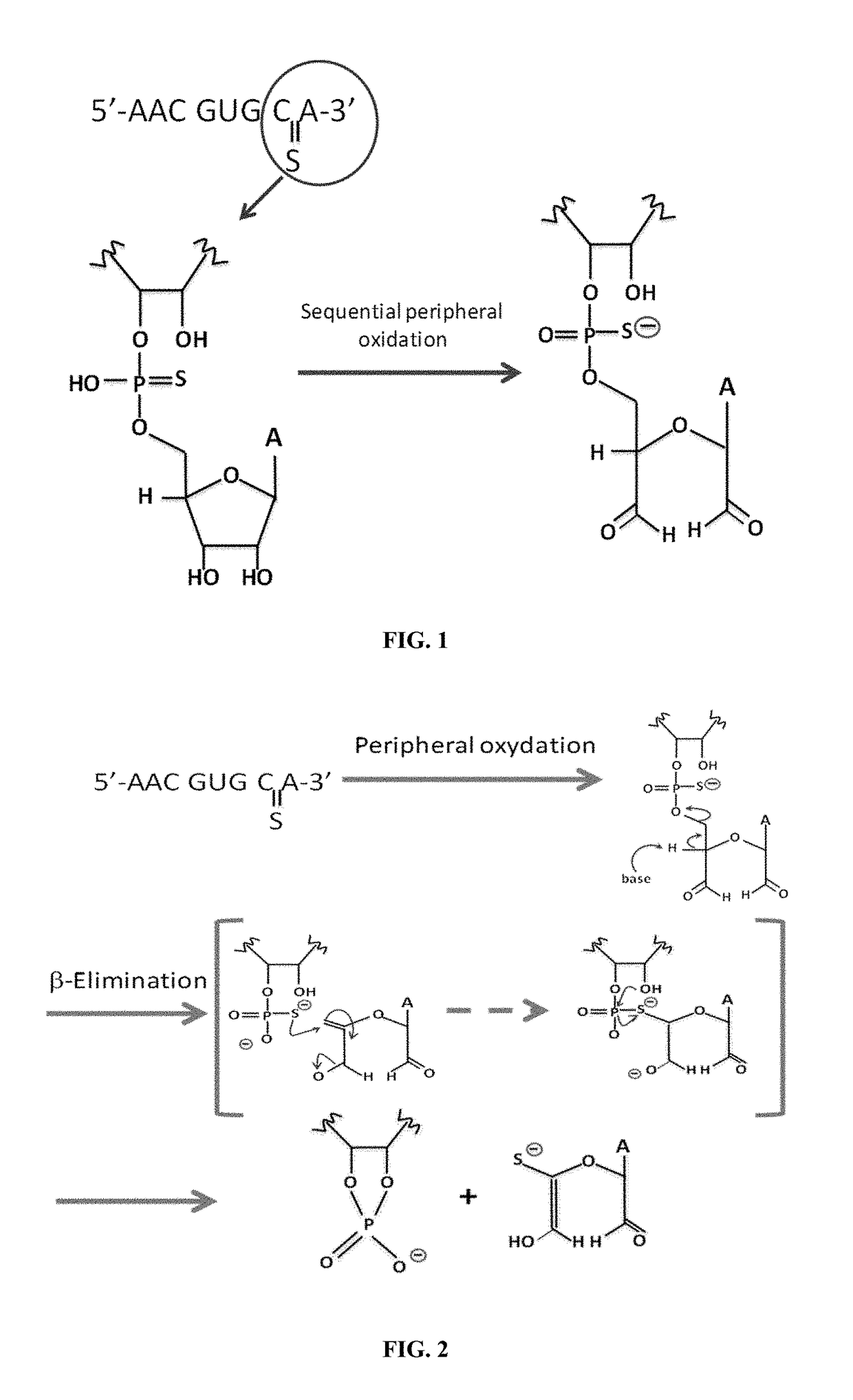
Sterile Formulation Comprising a Stable Phosphorothioate Oligonucleotide
a technology of phosphorothioate and oligonucleotide, which is applied in the direction of gene therapy, biochemistry apparatus and processes, pharmaceutical non-active ingredients, etc., can solve the problems of phosphorothioate stability problems, and phosphorothioate oligonucleotides are susceptible to desulfurization, so as to preserve the ability to inhibit irs-1 expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ed Stability Study of GS-101 in Presence of Phosphate
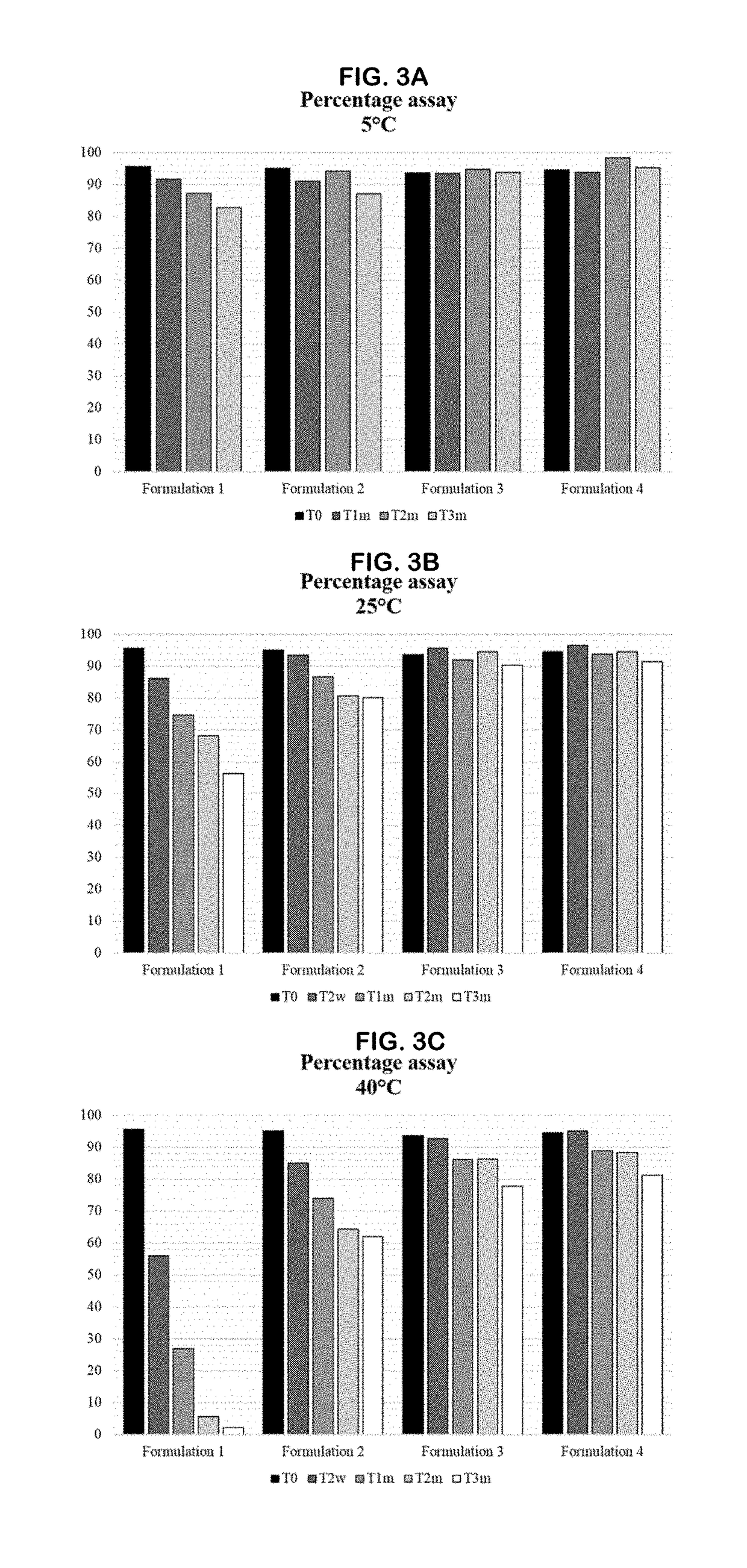
[0399]The accelerated stability study of GS-101 is reported in this example. A composition containing 0.86 mg GS-101 / g emulsion was evaluated in 7 different formulations, comprising phosphate compounds and / or an antioxidant, such as an agent comprising a thiol group. These formulations were kept at different storage conditions, and the stability of GS-101 in the different formulations was evaluated by reversed-phase high-performance liquid chromatography (RP-HPLC).
[0400]Materials and Methods
[0401]Preparation of the Sterile Bulk Emulsion
[0402]8% w / w* of Miglyol 812™, 3.5% w / w* of Gelot 64® and 2% w / w* of cetyl alcohol were mixed in a beaker. The beaker was then placed onto a magnetic stirrer-heater adjusted at 70° C. The resulting oil phase was solubilized and homogenized under continuous stirring (300 rpm) at 70° C. for 10 minutes.
[0403]Parallelly, 0.05% w / w* or 0.1% w / w* of Carbopol® 980NF (depending on the formulation as describ...
example 2
[0431]A long-term stability study of GS-101 was initiated and is reported in this example. The emulsion containing 0.86 mg GS-101 / g emulsion was evaluated in 5 different formulations, comprising phosphate and / or an agent comprising a thiol group. These formulations were kept at different storage conditions, and the stability of GS-101 in the different formulations was evaluated by RP-HPLC, as described in Example 1.
[0432]Materials and Methods
[0433]Preparation of the GS-101 Sterile Emulsions
[0434]The GS-101 sterile emulsions were prepared similarly to the emulsions of Example 1. These emulsions can contain one or more of the following substances:[0435]0.1% w / w* or 0.25% w / w* of N-acetylcysteine (NAC);[0436]0.436% w / w* of Na2HPO4.12H2O and 0.039% w / w* of NaH2PO4.H2O (15 mM phosphate final in the total composition);[0437]0.086% w / w* of GS-101 having the sequence SEQ ID NO: 2 (5′-TCTCCGGAGGGCTCGCCATGCTGCT-3′). * percentages are given in w / w of the total composition.
[0438]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com