Method for determining deletions in HBV pre-s2 region
a technology of deletion and hbv, which is applied in the field of detecting the pres2 deletion mutant large hepatitis b virus surface protein, can solve the problems of difficult detection of the pres2 mutant, labor-intensive cloning process, and high-risk markers for hcc incidence and recurrence not fully identified, so as to reduce the labor-intensive process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0049]The technical content of the present invention will become apparent by the detailed description of the following embodiments and the illustration of related drawings as follows.
[0050]Definitions
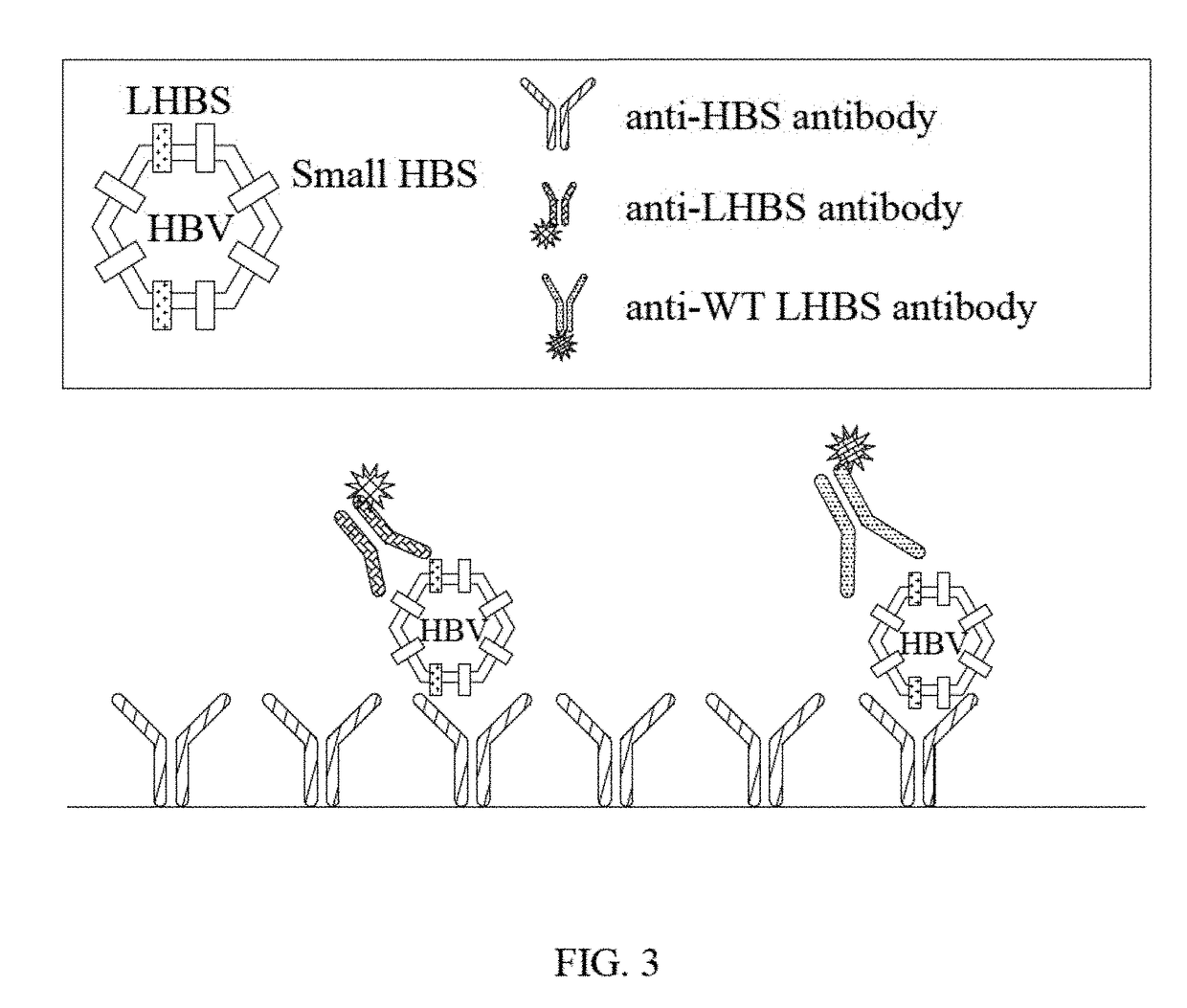
[0051]The term “HBS” as used herein refers to surface protein of hepatitis B virus (HBV). The HBS comprises large HBS, middle HBS and small HBS, which are different splicing forms of the surface protein.
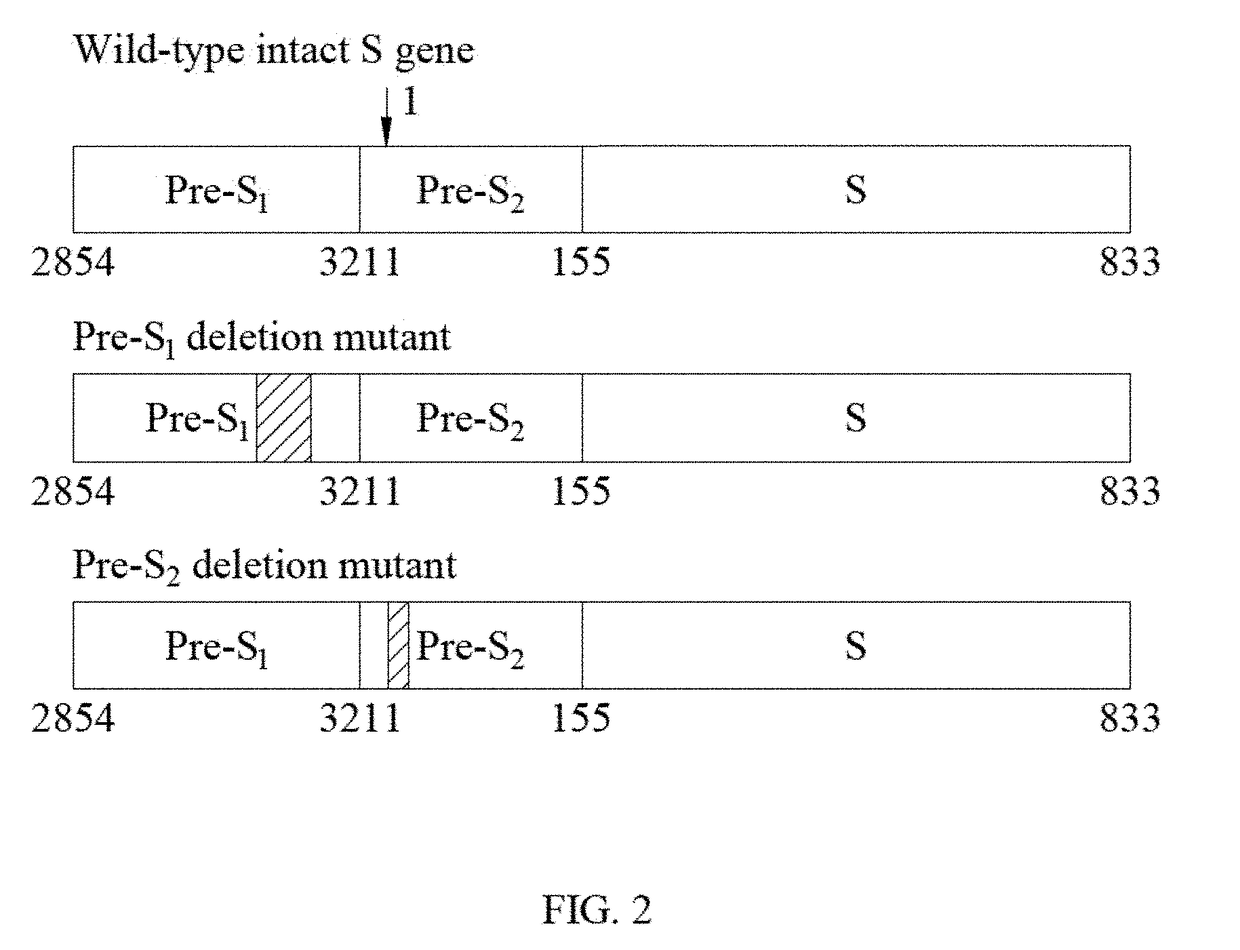
[0052]The term “LHBS” as used herein refers to large surface protein comprising pre-S1, pre-S2 and S regions.
[0053]The term “WT LHBS” as used herein refers to wild-type large surface protein comprising pre-S1, pre-S2 and S regions with a length of 401 amino acids.
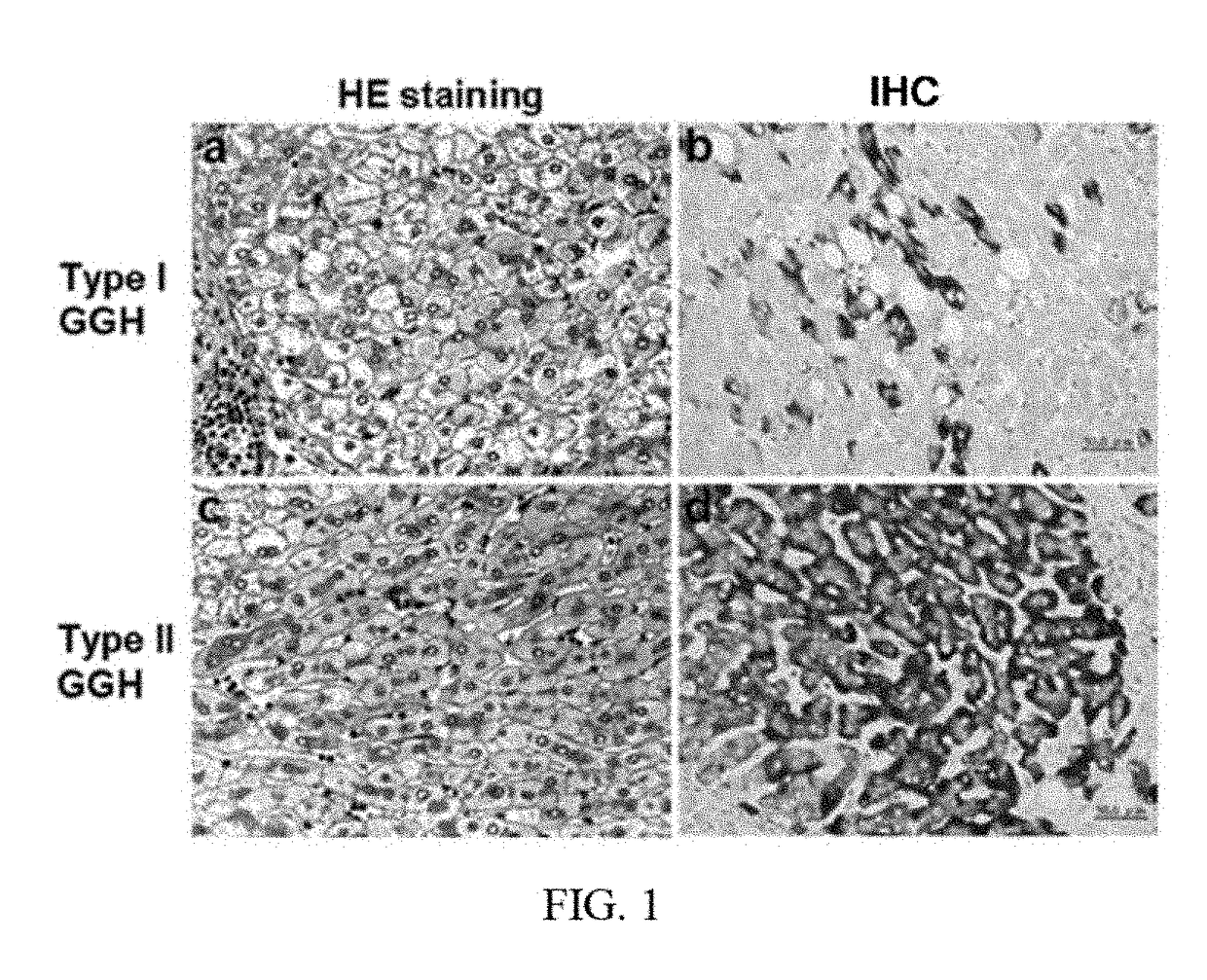
[0054]The term “pre-S2 deletion mutant LHBS” as used herein refers to large surface protein with a deletion around 20 amino acids in the pre-S2 region.
[0055]The term “detecting” is used in the broadest sense to include both qualitative and quantitative measurements of a target molecule. In one aspect, the detecting method as des...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com