Compounds comprising one or more hydrophobic domains and a hydrophilic domain comprising peg moieties, useful for binding cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Compounds of the Invention
[0315]The following compounds of the invention were synthesized:
Internal No.Scalechemical structure (modular)YieldBMO 29.89113110μMol5′-alphaTocopherolTEG-PEG2000-Fluos-3′ 58 pMol / μL-234 nMolScaleDMTrON-Synthesis / all 20 min. Coupling / Standard-CPG-Cleavage / DMTrOFF / Dialysis / no Purification / Crude product / Fluos-Conc.BMO 29.89113210μMol5′-Cholesteryl-TEG-PEG2000-Fluos-3′ 61 pMol / μL-216 nMolScaleDMTrON-Synthesis / all 20 min. Coupling / Standard-CPG-Cleavage / DMTrOFF / Dialysis / no Purification / Crude product / Fluos-Conc.BMO 29.89113310μMol5′-CholesterylTEG-CholesterylTEG-PEG2000-Fluos-3′ 43 pMol / μL-153 nMolScaleDMTrON-Synthesis / all 20 min. Coupling / Standard-CPG-Cleavage / DMTrOFF / Dialysis / no Purification / Crude product / Fluos-Conc.BMO 29.89113710μMol5′-CholesterylTEG-CholesterylTEG-PEG2000-BiotinTEG-3′111 pMol / μL-200 nMolScaleDMTrOFF-Synthesis / all 20 min. Coupling / Standard-CPG-Cleavage / Dialysis / no Purification / Crude product / Conc. estimatedBMO 29.89118010μMol5′-Ch...
example 4
Comparison of Compounds of the Invention Containing One vs. Two Hydrophobic Moieties
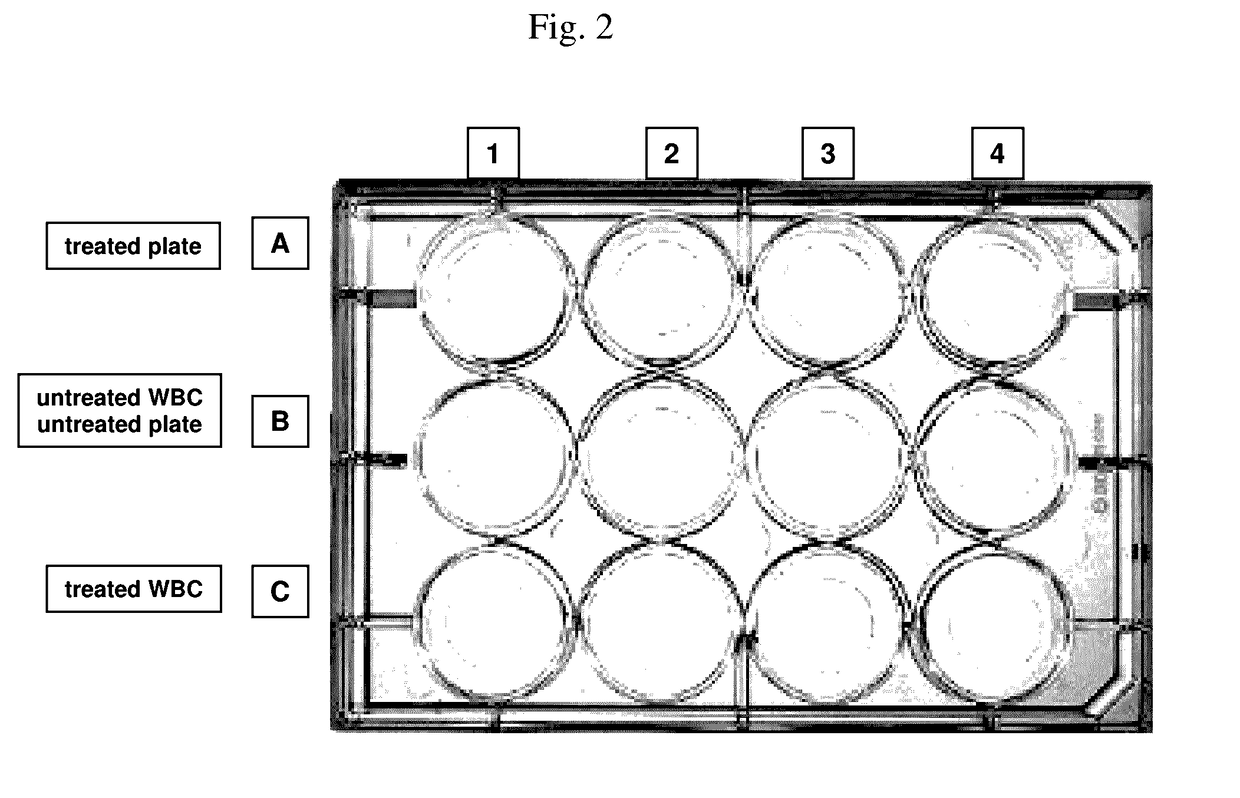
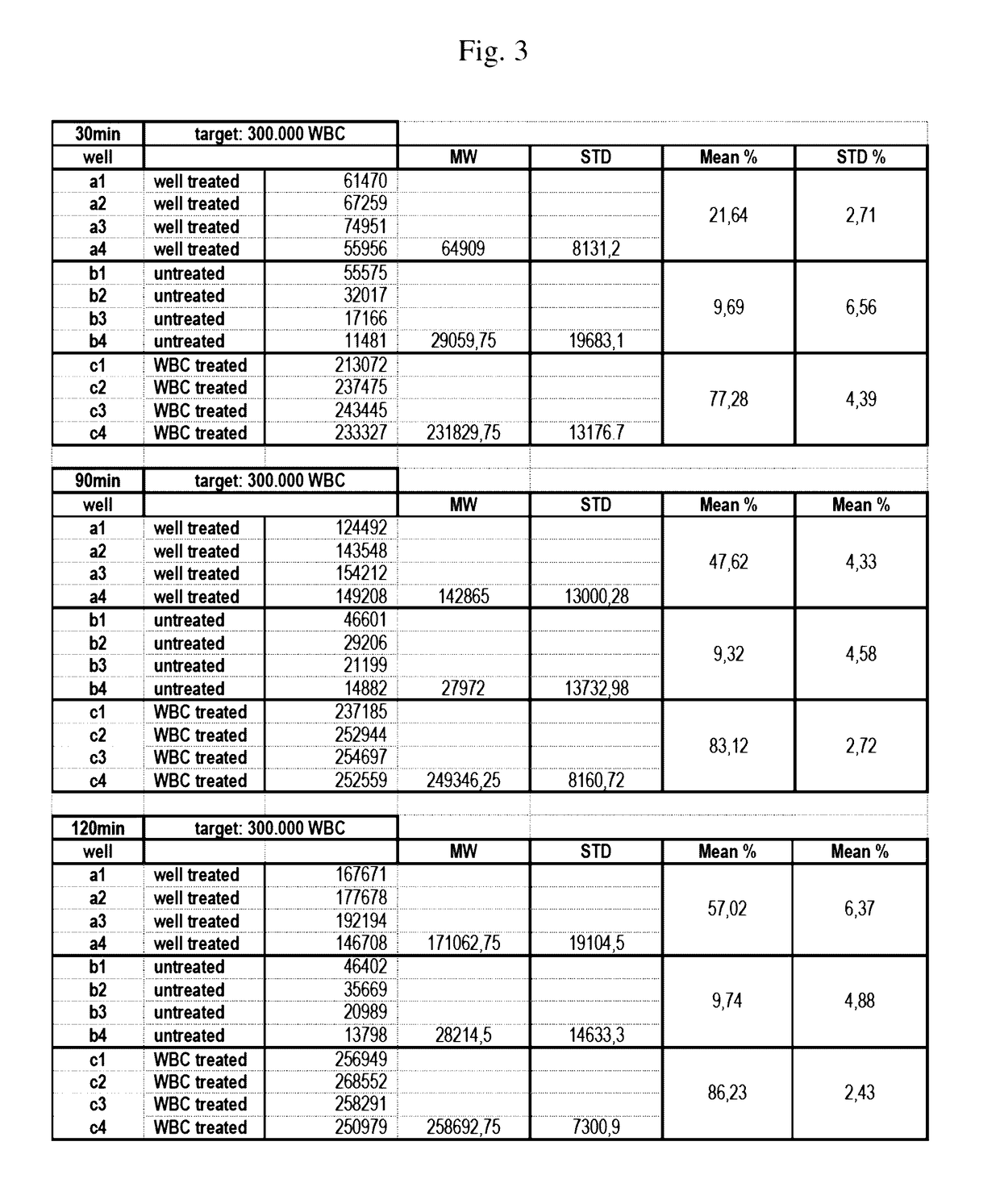
[0351]Aim of this experiment: Testing of the white blood cell immobilization on a streptavidin-coated surface using different molecules of the invention. In particular, the performance of a single cholesterol-molecule and different dual-linker molecules (i.e. containing two hydrophobic moieties) was tested. In detail, immobilization of white blood cells (WBCs) on a Streptavidin-coated surface using different linker molecules was tested on a 12-well plate: 300 000 WBCs / well. This was followed by the measurement of the cell recovery rate after immobilization and washing of the cells using the Cellavista instrument (10× Nuclei Operator s9s5).
InternalMolecule testedCharacteristicsNoStructure5′-(Cholesterol-TEG)1-Doubler-dT-Biotin-3′Mono-linker29.891272Y = Cholesteryl-TEGX = DoublerZ = dTBiotin5′-(Cholesteryl-TEG)2-Spacer C12-dT-Dual linker29.8912533′-YYXTZ-5′Biotin_TEG-3′ INVERSY = Cholesteryl-TEGX = Spa...
example 5
Stabilization of Cells Using Compounds of the Invention
[0356]The effect of compounds of the invention on stabilizing cells and on immobilization was determined.
[0357]A) WBC Recovery Rate After Centrifugation and Cell Immobilization Using Different Molecules
[0358]As shown in FIG. 14, molecule probes HH1749*, HH1750* and HH1755* (* Biotin-PEG-Lysin-(C18)2) show different performance concerning recovery rate after centrifugation: The higher the concentration of the molecule, the higher the cell recovery rate after centrifugation. Centrifugation characteristics: 10 min, 300×g. As can be seen from FIG. 15, molecule probes HH1749*, HH1750* and HH1755* show different performance concerning cell immobilization rate at different concentrations. The higher the linker concentration, the higher the cell immobilisation rate.
[0359]B) WBC Recovery Rate After Centrifugation Using Different Linkers—Different Points of Time
[0360]As can be seen from FIG. 16, molecules A and B (A: Cholesteryl-TEG-Chole...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com