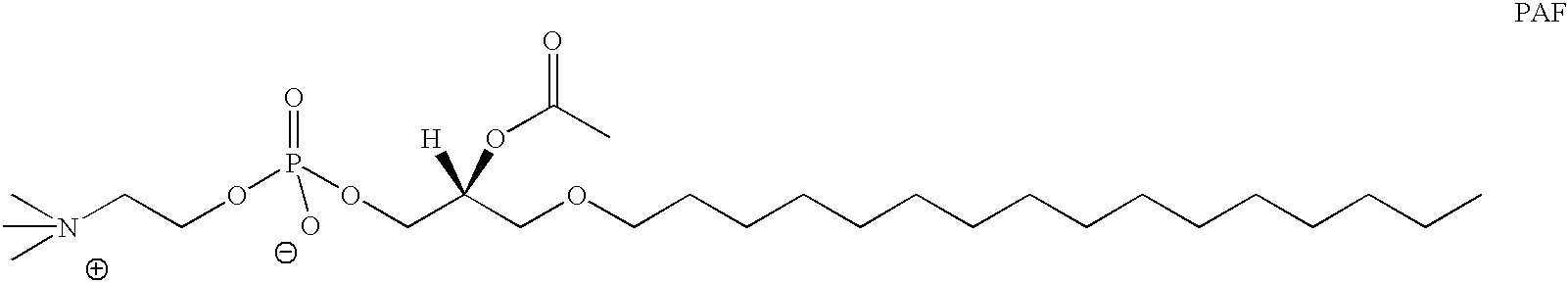
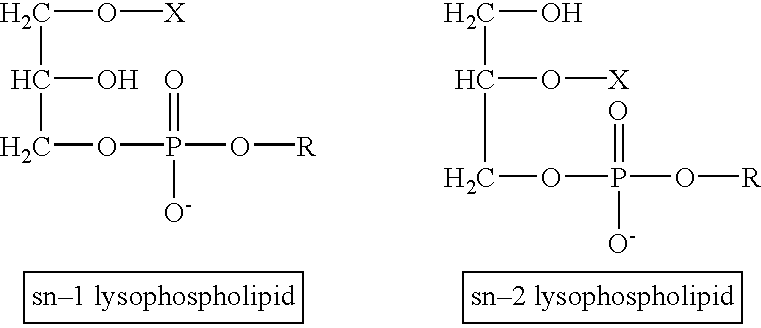
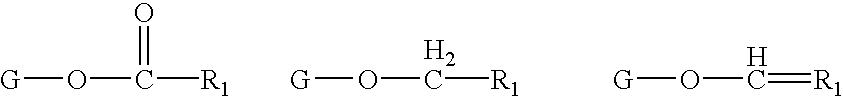
Determination of biological characteristics of embryos fertilized in vitro by assaying for bioactive lipids in culture media
a technology of biological characteristics and culture media, which is applied in the direction of biochemistry apparatus and processes, instruments, enzymology, etc., can solve the problems of multiple pregnancies, emotional and economic problems in family planning, and many couples are not able to conceive in the traditional fashion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Assay of Media Specimen from In vitro Fertilization Culture to Measure Total Lysophospholipid (LPX) and Total Glycerophosphatidyl Compounds (GPX) by a Redox-Coupled Enzymatic Reaction
[0050] IVF embryo conditioned culture media specimens were obtained from freshly cultured human embryos. Embryos were cultured in a media blend, containing RPMI media (from Irvine Scientific, Santa Ana, Calif.) as a base, with supplemental factors added. A control sample of the media blend was also obtained for comparison. Embryos were cultured in 0.1 ml media under a drop of oil to prevent evaporation. Embryos were maintained at 37.degree. C. in a humidified incubator. Media samples were collected by aspiration from individually cultured human IVF embryos at 72 hours of development. Additional media was added to the embryo culture if in vitro development were to be continued. The aspirated media specimen was either processed immediately or stored at -20.degree. C. or below until processing.
[0051] Reage...
example 2
Assay of Media Specimen from In vitro Fertilization Culture to Measure Lysophosphatidic Acid (LPA) and Glycerol-3-phosphate (G3P) By a Redox-Coupled Enzymatic Reaction
[0059] IVF embryo conditioned culture media were obtained from freshly cultured human embryos as described in Example #1.
[0060] Reagents
[0061] Lysophospholipase (LYPL) was purchased from Asahi Chemical (Tokyo, Japan). Glycerol-3-phosphate oxidase (GPO) was purchased from Toyobo (Osaka, Japan). 4-aminoantipyrine (AAP) was purchased from Sigma Chemical Co., St. Louis, Mo. Glycerol-3-phosphate dehydrogenase (GDH), peroxidase (POD) and beta-Nicotinamide-adenine dinucleotide (NADH) were purchased from Roche (Boehringer Mannheim, Indianapolis, Ill.). 3,5-Dichloro-2-hydroxybenzenesulfonic acid (HDCBS) was purchased from Biosynth (Naperville, Ill.). Amplex Red reagent (10-acetyl-3,7-dihydroxyp-henoxazine) was purchased from Molecular Probes, Inc. (Eugene, Oreg.). All lipid or glycerophosphatidyl standards were purchased from A...
example 3
Liquid Chromatography--Mass Spectrometry Analysis of Bioactive Lipids in Embryo Culture Media
[0068] IVF embryo conditioned culture media are obtained from freshly cultured human as described in Example #1.
[0069] Reagents
[0070] Methanol, chloroform and hydrochloric acid, are purchased from Fisher Scientific. All lipid or glycerophosphatidyl standards are purchased from Avanti Polar Lipids, Alabaster, Ala. or Sigma Chemical Co. LC-MS-MS triple quadrapole instrument is a Quattro Ultima (MicroMass, Beverly, Mass.). C18 columns are purchased from Keystone Scientific (Bellefonte, Pa.).
[0071] Sample Preparation
[0072] IVF media samples are diluted in methanol in preparation for analysis on the LC-MS. Samples are diluted 1:10 by combining 10 .mu.l of each sample with 90 .mu.l methanol. Samples are then centrifuged to remove precipitated protein and placed into maximum recovery autosampler vials. Alternatively, samples are extracted using methanol:chloroform (2:1), dried under nitrogen gas, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com