Methods and related compositions for the treatment of cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials
[0043]1,2-Disteroyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-2000] (PEGPE) was from Avanti Polar Lipids (Alabaster, Ala., USA). pNp-PEG3400-pNP was purchased from Laysan Bio (Arab, Ala.). Curcumin (CUR), vitamin E and triethylamine (TEA) were purchased from Sigma (St. Louis, Mo., USA). Doxorubicin HCl (DOX) was from LC Laboratories (Woburn, Mass.). Accumax was from Innovative Cell Technologies, Inc. (San Diego, Calif.). The CellTiter-Glo luminescent cell viability assay kit was from Promega (Fitchburg, Wis.). All other reagents were of analytical grade. DOX free base was obtained by incubating DOX HCl (in methanol, 0.5 mg / ml) with 5-fold molar excess of TEA overnight. pNP-PEG3400-PE was synthesized in-house.
example 2
Micelle Preparation
[0044]DOX and / or CUR drug-loaded mixed micelles were prepared by the thin film hydration method. CUR (1 mg / ml in 0.1% acetic acid-methanol solution) and / or DOX free base (0.5 mg / ml in methanol solution) were added to PEG2000-PE solution in chloroform. Initial drug amounts were adjusted after the formulation optimization studies to result in CUR:DOX ratio of 32 (w / w). The organic solvents were removed by the rotary evaporation and a thin film of drugs / micelle-forming material mixture was formed. This film was further dried overnight in freeze-dryer to remove any residuals of organic solvents (Freezone 4.5 Freeze Dry System, Labconco, Kansas City, Mo.). Drug-loaded micelles were formed by resuspending the film in phosphate buffered saline (PBS) pH 7.2, to give the final concentration of micelle forming materials of 5 mM in all formulations. The micelle formulations were dialyzed 1 h against PBS pH 7.2 to remove excess of TEA. Excess non-incorporated drugs were separ...
example 3
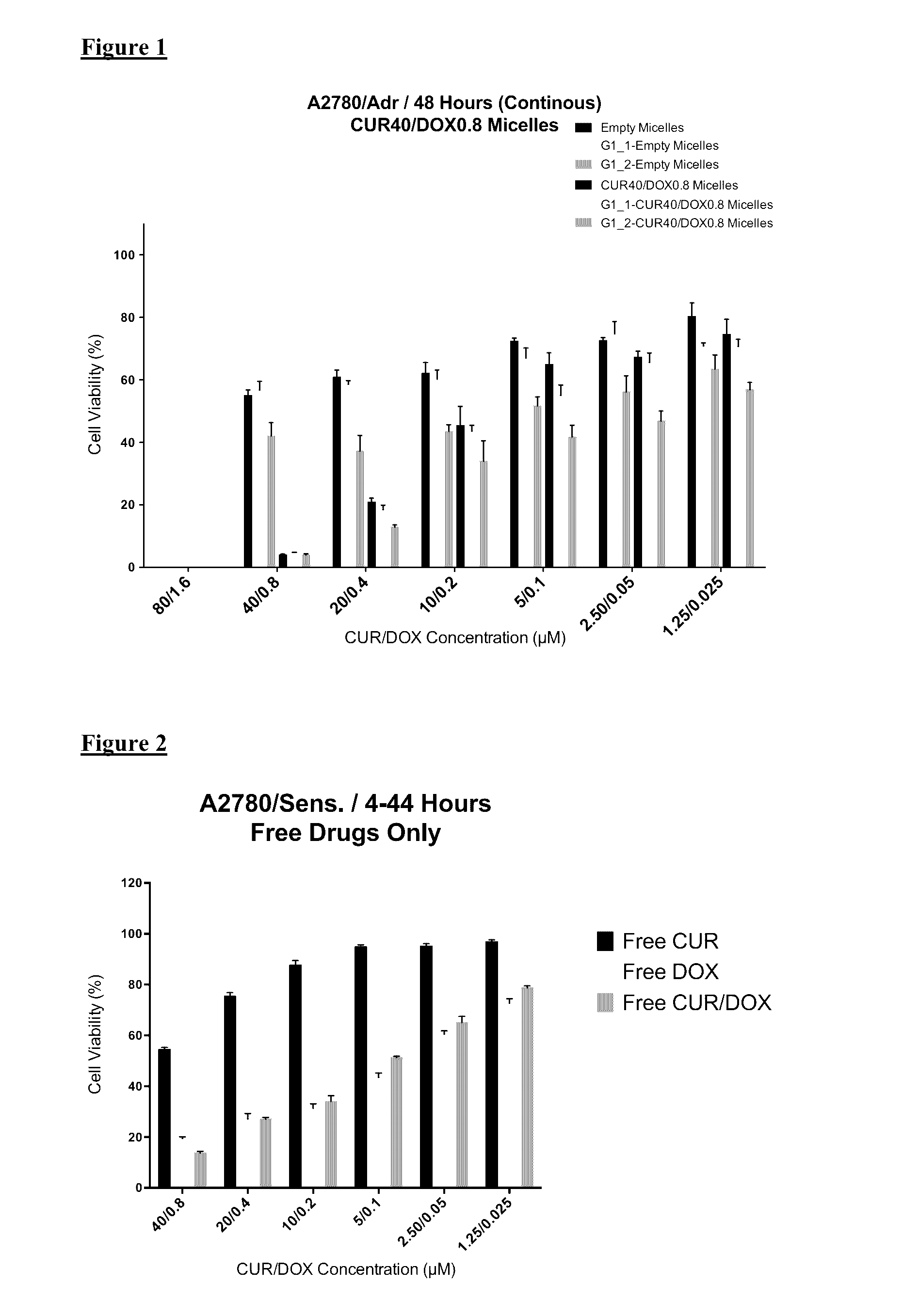
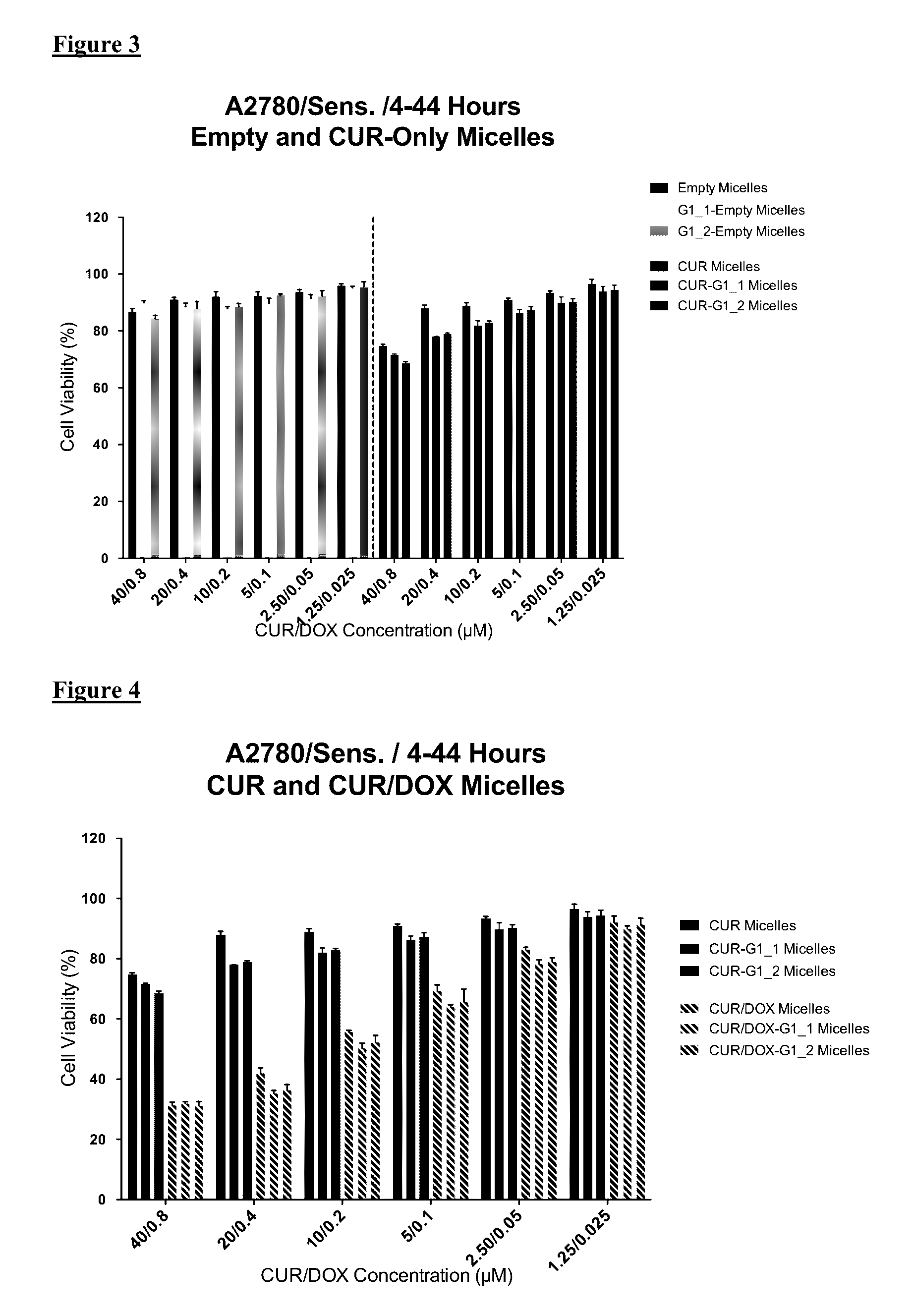
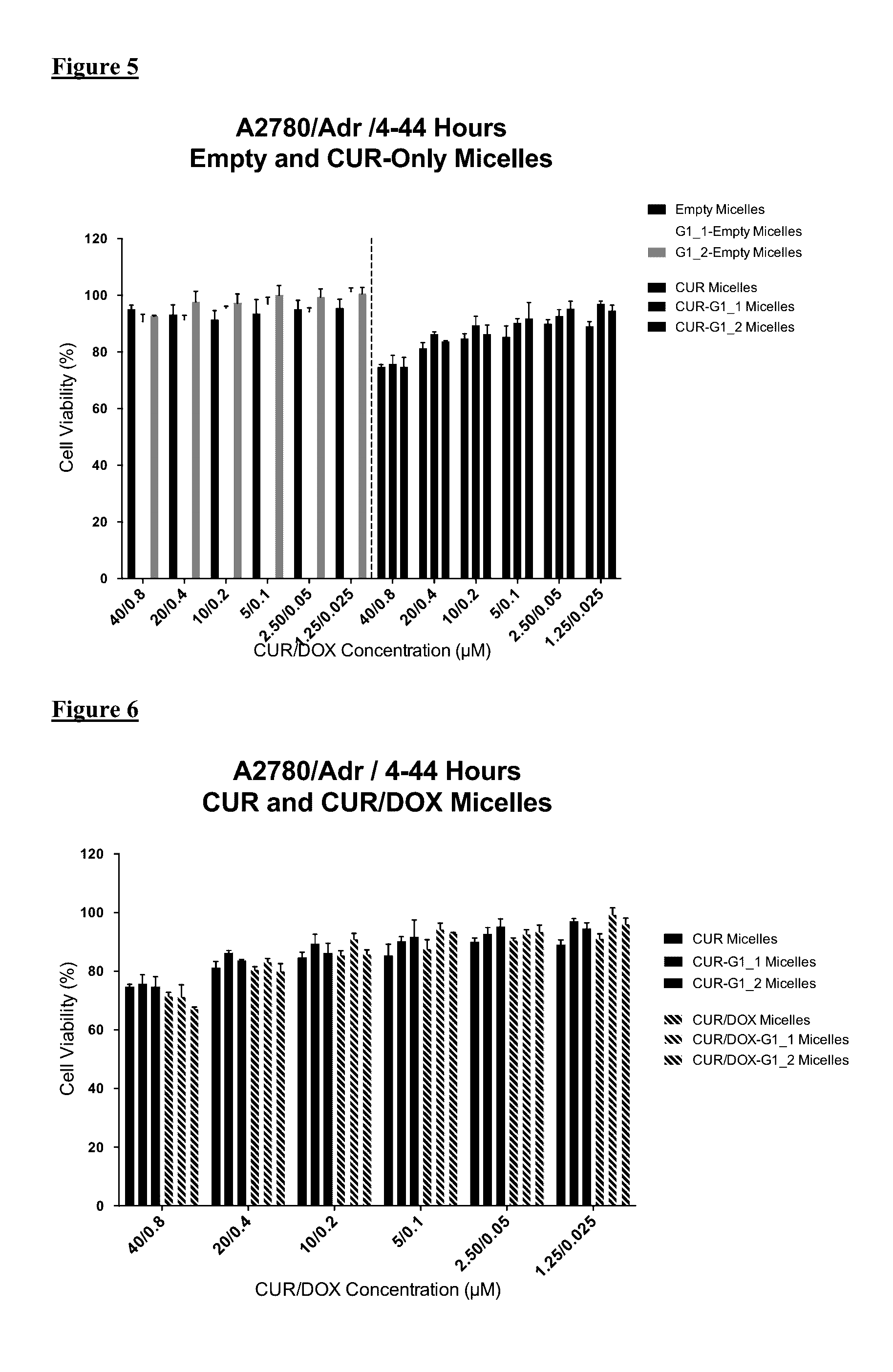
GLUT-1 scFv Modified Micelle Cytotoxicity on Ovarian Cancer Cell Line
[0046]For cytotoxicity evaluations, A2780 human ovarian carcinoma (ATCC) and its Doxorubicin resistant derivative A2780 / Adr (ECACC) were used. A2780 cells were cultured in RPMI medium supplemented with 10% FBS and 1% penicillin-streptomycin. Drug resistant ovarian cancer cells were grown in same medium with the addition of 100 nM DOX.
[0047]The cells were seeded in 96-well plates at a density of 3000 cells / well or 5,000 cells / well for 48 or 24 hours treatment groups, respectively. The cells were treated with free drugs or micelle formulations containing 40 μM CUR and 0.8 μM DOX in serum complete RPMI medium. The cells were incubated 24 h and 48 h continuously in the continuous treatment groups. Additionally, in another treatment regimen the cells were incubated with drugs / micelles for 4 hours then washed and further incubated for 44 h. At the end of treatment times, the wells were washed twice with medium and then i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com