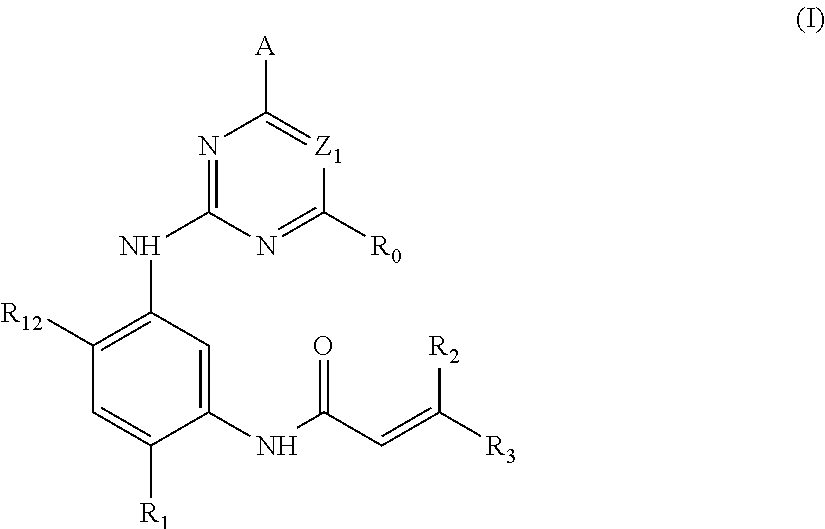
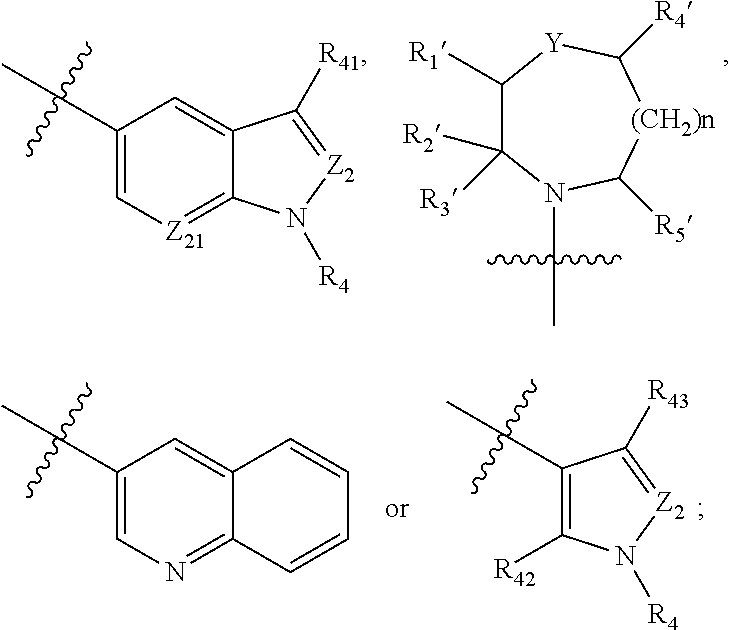
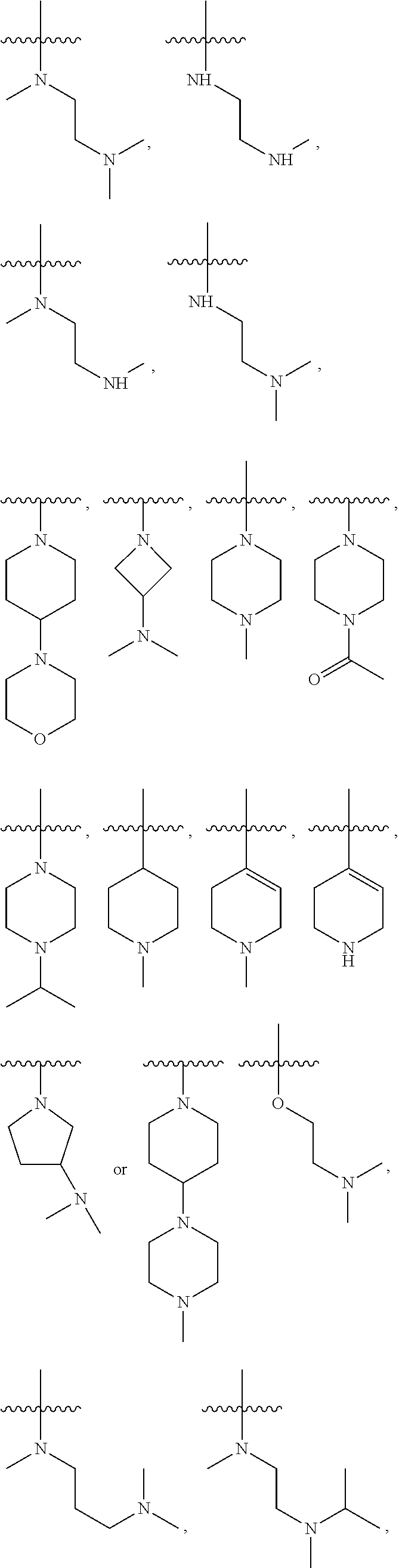
2,4-disubstituted phenylene-1,5-diamine derivatives and applications thereof, and pharmaceutical compositions and pharmaceutically acceptable compositions prepared therefrom
a technology of phenylene and diamine, which is applied in the field of medical technology, can solve the problems of poor effect of the second-generation inhibitor on drug resistant patients and no drugs available for them
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N-(5-(5-chloro-4-(1-methyl-1H-pyrrolo[2,3-b]pyridin-5-yl)pyrimidin-2-amino)-2-((2-(dimethylamino)ethyl)methylamino)-4-methoxyphenyl)acrylamide (compound Z-1)
[0149]
Step 1
5-bromo-1-methyl-1H-pyrrolo[2,3-b]pyridine (Compound 1-2)
[0150]At 0° C., a solution of compound 5-bromo-1H-pyrrolo[2,3-b]pyridine (5 g, 25.4 mmol, commercially available) in DMF was added to a solution of sodium hydride (4.32 g, 30.5 mmol) in 40 ml of DMF, and vigorously stirred at 0° C. for 0.5 h, and then methyl iodide (1.22 g, 30.5 mmol) was added, and stirred vigorously at room temperature for 3 h. The reaction progress was monitored by TLC. After completion of the reaction, the reaction solution was poured into ice water. After the solids were completely precipitated, the solids were filtered and dissolved in dichloromethane and methanol (10:1), washed with water three times, and dried. The organic layer was separated, and concentrated under reduced pressure to give compound 1-2 (5.4 g, 99%). The product was use...
example 2
N-(5-(5-chloro-4-(1-methyl-1H-pyrrolo[2,3-b]pyridin-5-yl)pyrimidin-2-amino)-2-(4-(dimethylamino)piperidin-1-yl)-4-methoxyphenyl)acrylamide (compound Z-2)
[0157]
Step 1
5-chloro-N-(4-(4-(dimethylamino)piperidin-1-yl)-2-methoxy-5-nitrophenyl)-4-(1-methyl-1H-pyrrolo[2,3-b]pyridin-5-yl)pyrimidin-2-amine (compound 2-6)
[0158]Compound 1-5 (260 mg, 0.607 mmol) and potassium carbonate (251 mg, 1.822 mmol) were added into a 25 ml reaction flask, DMF (10 mL) was added to partially dissolve the substrate, and then N,N-dimethylamino piperidine (85.4 mg, 0.667 mmol) was added, and the reaction system was maintained at 70° C. for 3 h. The reaction progress was monitored by TLC. After completion of the reaction, the reaction solution was extracted with EA / water system for three times, and the organic layer was separated, washed with water, saturated brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure to give the crude compound 2-6, 220 mg, yield 68%. MS M / Z (ESI) 537.2[M+H]+.
St...
example 3
N-(5-(5-chloro-4-(1-methyl-1H-pyrrolo[2,3-b]pyridin-5-yl)pyrimidin-2-amino)-4-methoxy-2-(4-methylpiperazin-1-yl)phenyl)acrylamide (compound Z-3)
[0161]
Step 1
5-chloro-N-(2-methoxy-4-(4-methylpiperazin-1-yl)-5-nitrophenyl)-4-(1-methyl-1H-pyrrolo[2,3-b]pyridin-5-yl)pyrimidin-2-amine (compound 3-6)
[0162]N-methylpiperazine (126 mg, 1.26 mmol) and potassium carbonate (261 mg, 1.89 mmol) were added to a solution of compound 1-5 (200 mg, 0.47 mmol) in 4 ml of DMF, and vigorously stirred at 100° C. for 2 h. The reaction progress was monitored by TLC. After completion of the reaction, 10 ml of water was added, extracted with EA / water system for three times, and the organic layer was separated, and concentrated under reduced pressure to give compound 3-6 (200 mg, 84%). MS m / z (ESI): 509.2[M+H]+.
Step 2
N1-(5-chloro-4-(1-methyl-1H-pyrrolo[2,3-b]pyridin-5-yl)pyrimidin-2-yl)-6-methoxy-4-(4-methylpiperazin-1-yl)benzene-1,3-diamine (compound 3-7)
[0163]By using compound 3-6 (200 mg, 0.39 mmol) as the s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com