Emulsions or microemulsions for use in endoscopic mucosal resectioning and/or endoscopic submucosal dissection
a technology of endoscopic submucosal dissection and emulsion, which is applied in the direction of oesophagoscope, packaged goods, packaged foodstuffs, etc., can solve the problems of difficult to produce the proper submucosal fluid cushion and maintain the desired height, and the solution tends to dissipate quickly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
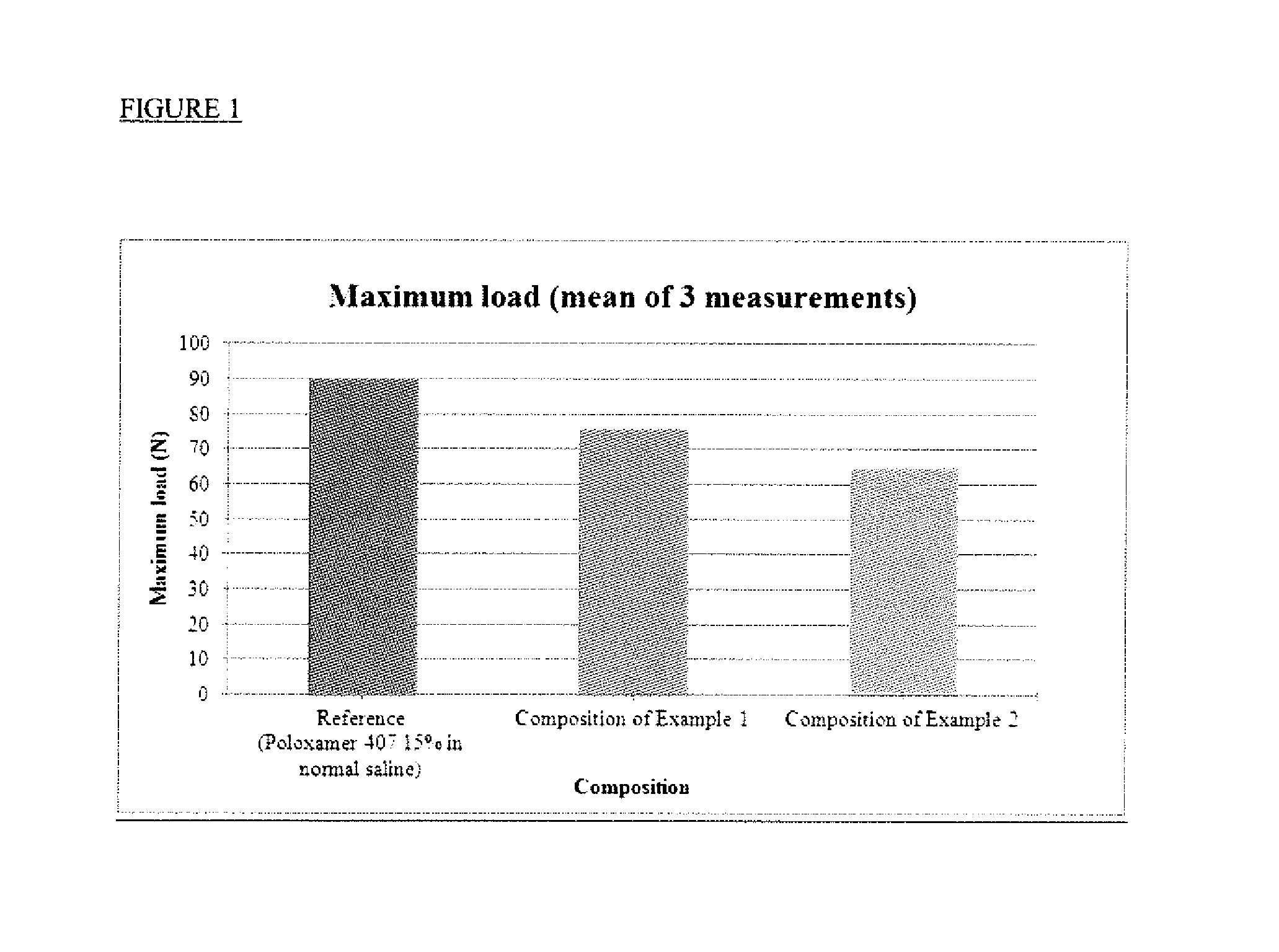
Image
Examples
example 1
[0098]
Componentg / 100 gMethylene blue0.0010Sodium chloride0.9000Poloxamer 40715.0000Soybean oil0.0800Glycerol0.0025Egg lecithin0.0120Sodium oleate0.0003Water for injectionq.s. 100.0g
[0099]The manufacture of the composition is described hereinafter (for 10.00 Kg of final composition):
a. In a suitable vessel provided with a stirrer, about 8000 mL of water for injection are loaded; then, 90.00 g of sodium chloride are added. The mixture is kept under stirring until a complete dissolution is achieved. The obtained solution is cooled at a temperature ranging between 5° C. and 10° C.; then, 1500.00 g of poloxamer 407 are added under stirring. The mixture is kept under stirring until a complete dissolution is achieved.
b. In a suitable vessel provided with a stirrer, about 181 mL of water for injection are loaded; the temperature is raised at 80° C. 1.20 g of egg lecithin, 0.25 g of glycerol, 0.03 g of sodium oleate are added under stirring. The stirrer is operated until a complete homogeniz...
example 2
[0100]
Componentg / 100 gMethylene blue0.0010Sodium chloride0.9000Poloxamer 40715.0000Soybean oil0.1600Glycerol0.0050Egg lecithin0.0240Sodium oleate0.0006Water for injectionq.s. 100.0g
[0101]The composition was obtained by a process similar to that described in Example 1.
example 3
[0102]
Componentg / 100 gMethylene blue0.0010gSodium chloride0.9000gL-Glutamic acid1.0000gPoloxamer 18818.000gSoybean oil0.1600gGlycerol0.0050gEgg lecithin0.0240gSodium oleate0.0006gSodium hydroxideq.s. to bring the pH within 5.0 and 7.0Water for injectionq.s. to 100.0g
[0103]The manufacture of the composition is described thereinafter (for 10.00 Kg of final composition):
a) In a suitable vessel provided with a stirrer, about 6000 mL of water for injection are loaded; then, 90.00 g of sodium chloride are added. The mixture is kept under stirring until a complete dissolution is achieved. The obtained solution is cooled at a temperature ranging between 5° C. and 10° C.; then, 1800.00 g of poloxamer 188 are added under stirring. The mixture is kept under stirring until a complete dissolution is achieved.
b) In a suitable vessel provided with a stirrer, about 181 mL of water for injection are loaded; the temperature is raised at 80° C. 2.40 g of egg lecithin, 0.50 g of glycerol, 0.06 g of sod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com