Beta-catenin targeting peptides and uses thereof
a technology of beta-catenin and peptides, which is applied in the direction of peptide/protein ingredients, peptide sources, drug compositions, etc., can solve the problems of lack of binding affinity and/or specificity, poor cell permeability, etc., and achieve the effect of increasing the helical character of peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
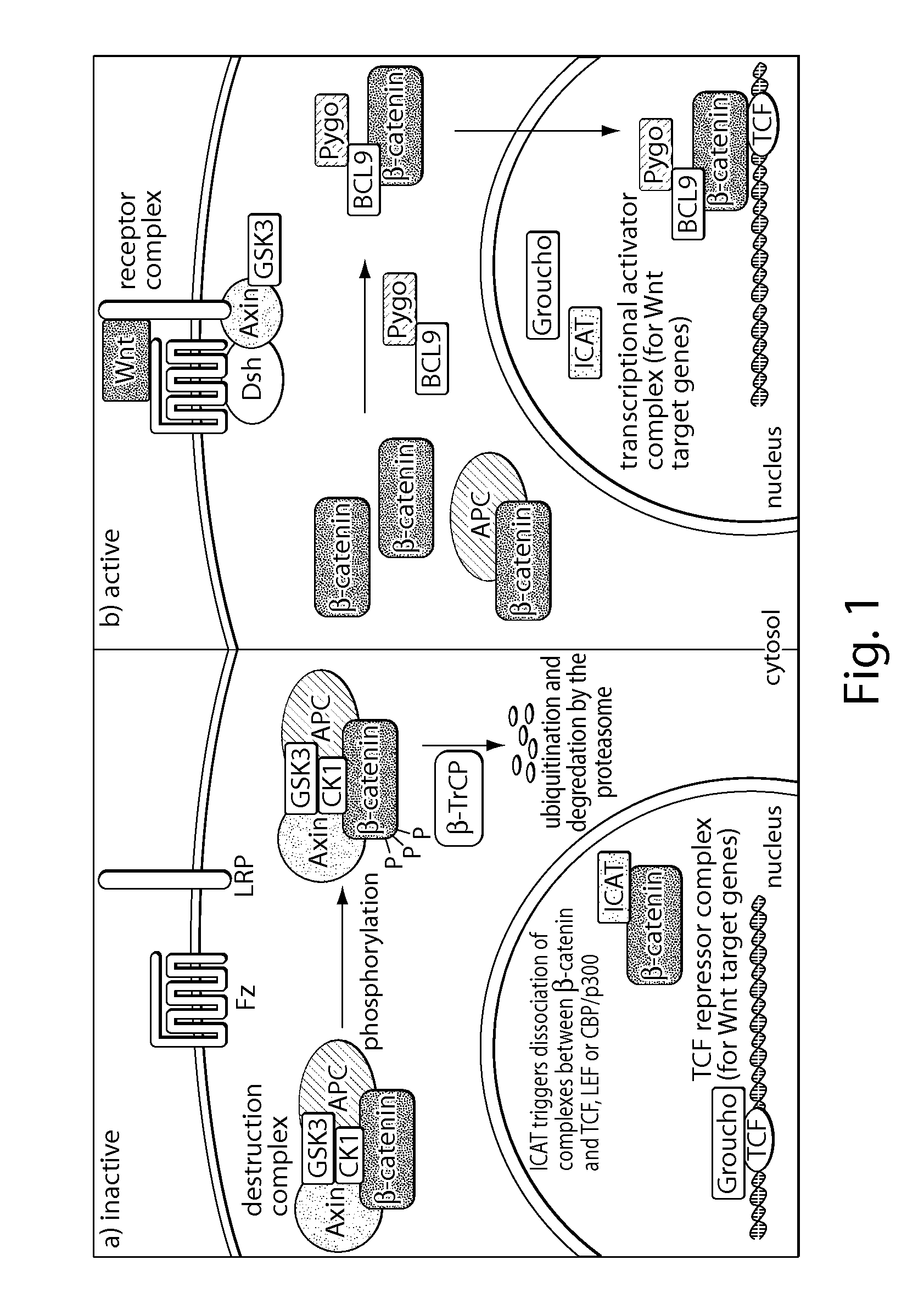
[0405]Deregulation of Wnt / β-catenin signaling is associated with several types of cancer.
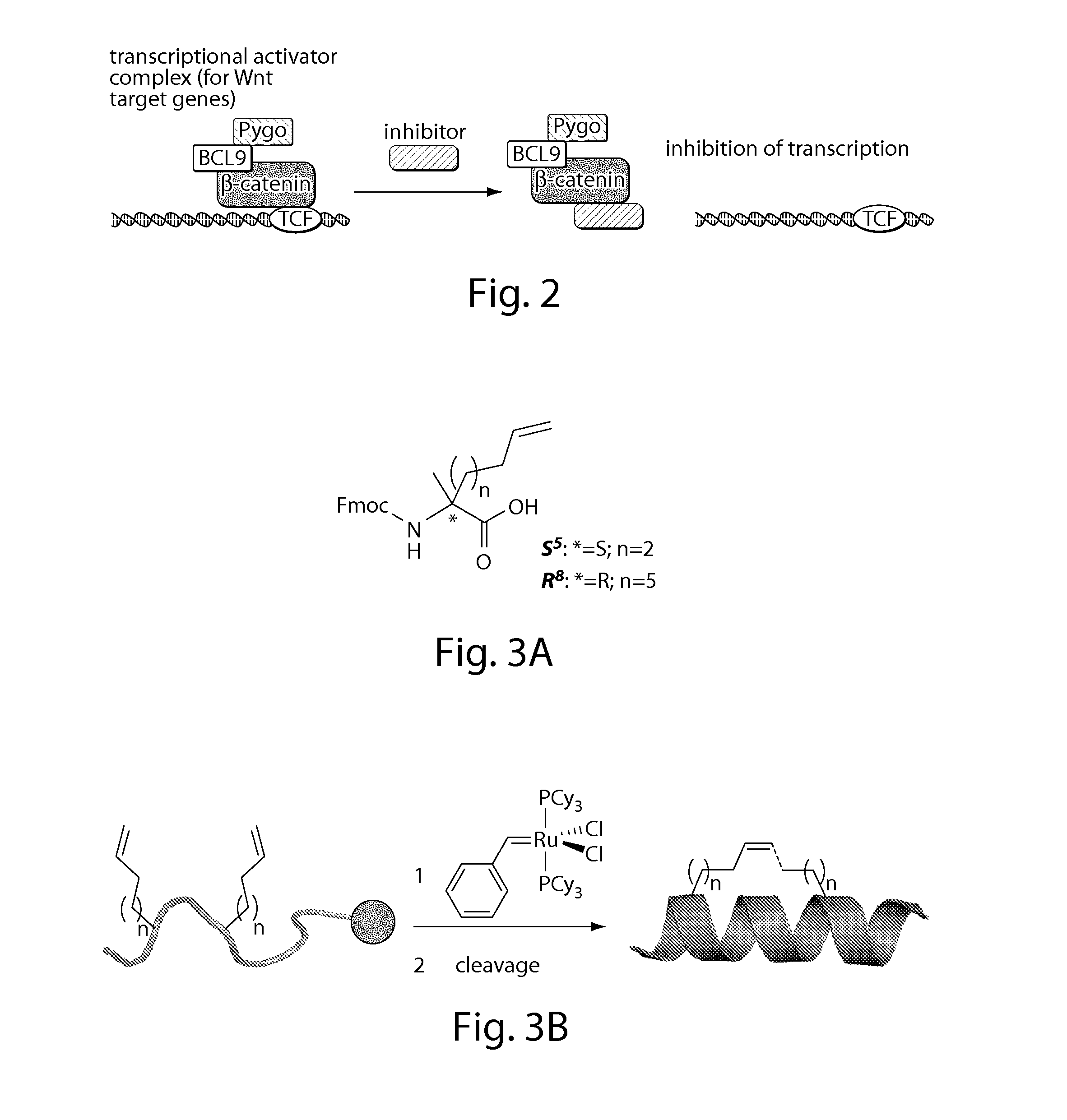
[0406]Especially, a reduced ability to degrade β-catenin, e.g., by the inactivation of the destruction complex or by loss of β-catenin phosphorylation sites, can be involved in the formation of cancer.[2, 3] Restoring this ability would contribute to target cancers that depend on deregulated Wnt signaling to grow. The manipulation of requisite protein-protein interactions offers a strategy to interfere with this pathway, e.g., targeting the signaling cascade downstream by inhibiting the interaction of β-catenin with TCF transcription factors (FIG. 2). This setup may allow for inhibition of Wnt signaling independent of the site of deregulation within the pathway.
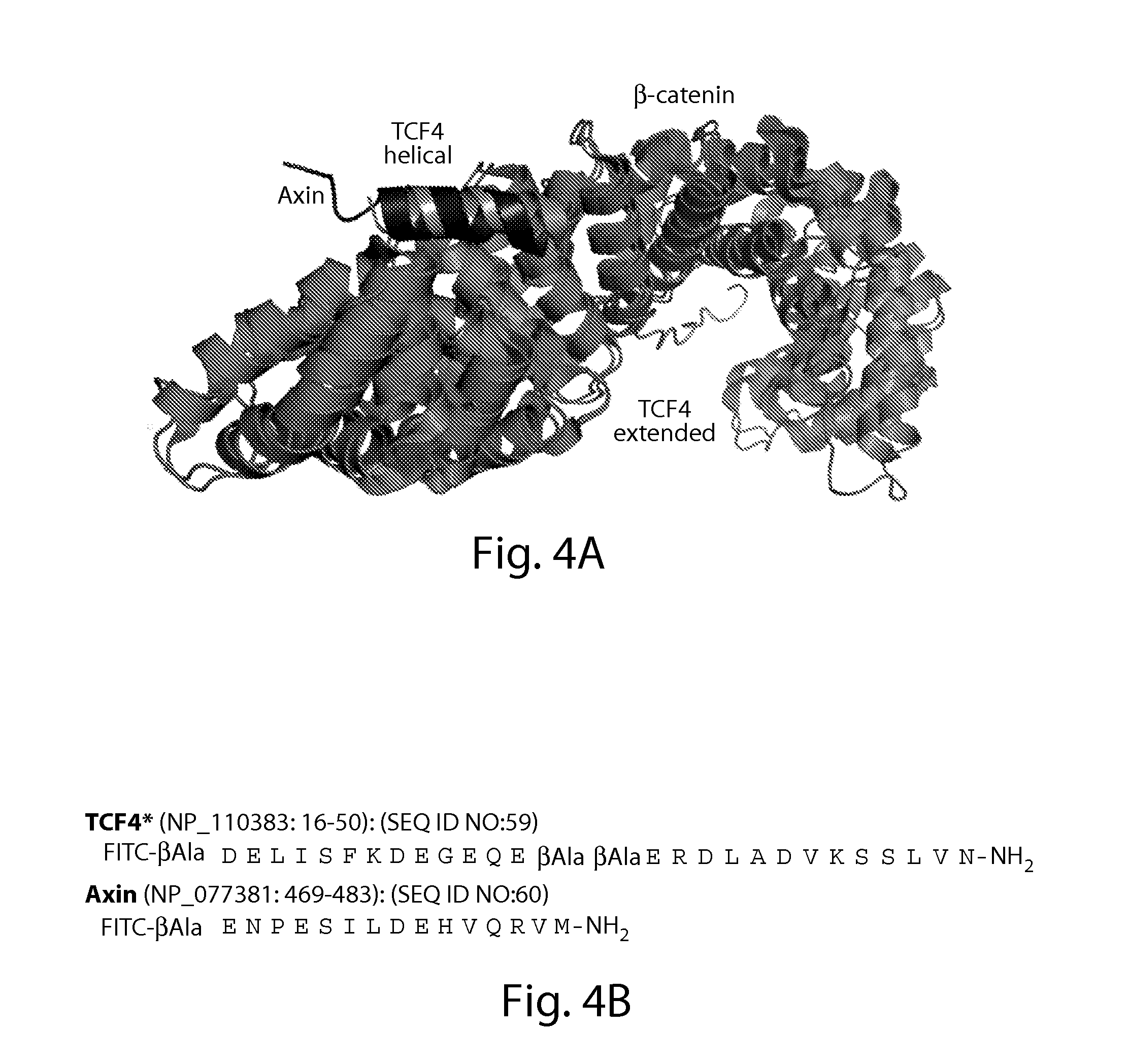
[0407]Due to large interaction areas, the precise manipulation of protein-protein interactions is complicated.[6] In principle, an isolated bioactive helix of a protein could be used to interfere with such interactions. However, small pep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com