Histidine Engineered Light Chain Antibodies and Genetically Modified Non-Human Animals for Generating the Same
a technology of light chain antibody and histidine, which is applied in the field of histidine engineered light chain antibody and genetically modified non-human animals for generating the same, can solve the problems of bispecific antibody having a suitable light chain component that can satisfactorily associate with each of the heavy chains of the bispecific antibody, requiring high doses to achieve the desired effect, and reducing binding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
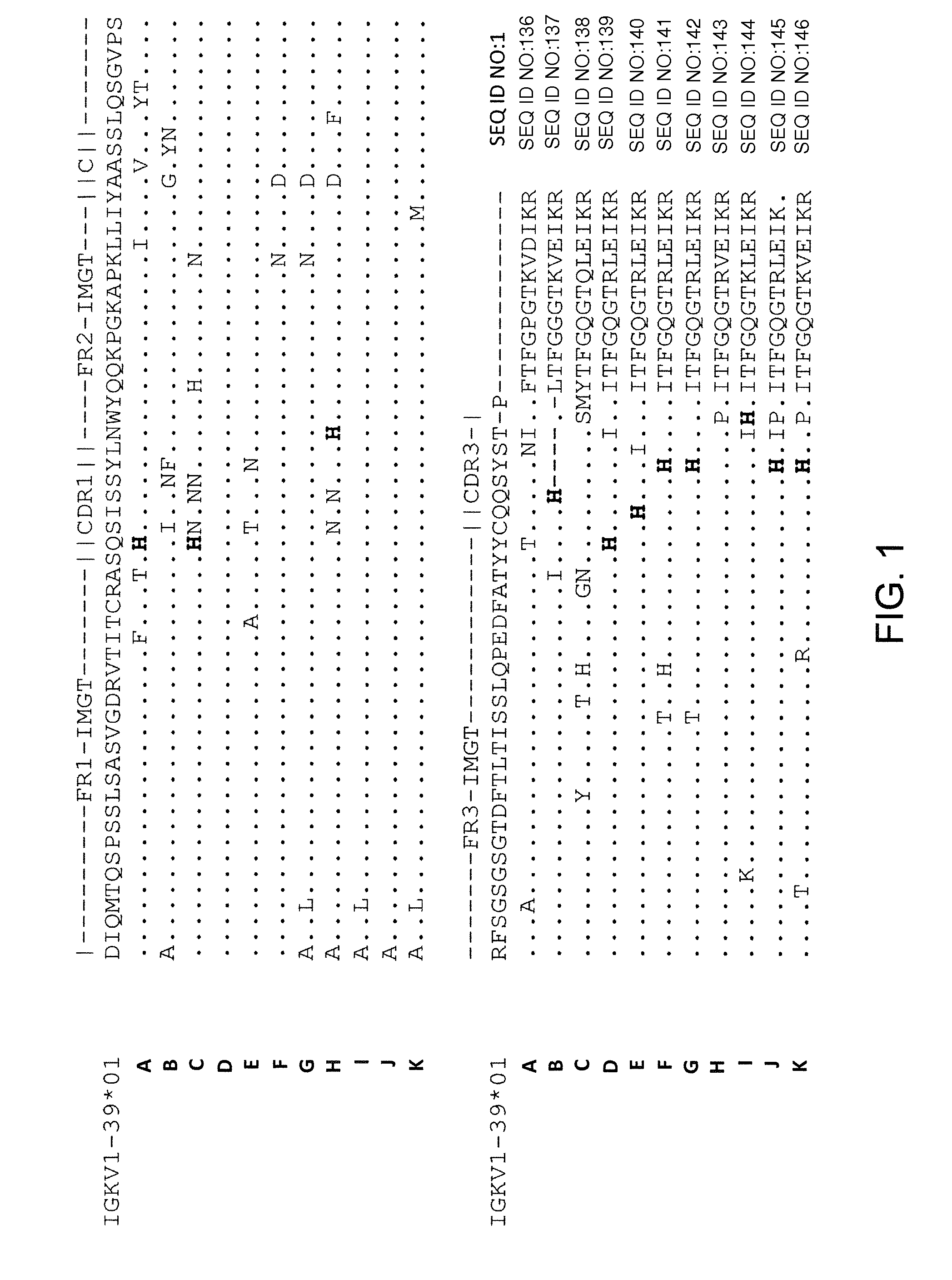
Identification of Histidine Residues in Antigen-Specific Human Light Chains
[0259]Generation of a common light chain mouse (e.g., Vκ1-39 or Vκ3-20 common light chain mouse) and antigen-specific antibodies in those mice is described in, e.g., U.S. patent application Ser. Nos. 13 / 022,759, 13 / 093,156, and 13 / 412,936 (Publication Nos. 2011 / 0195454, 2012 / 0021409, and 2012 / 0192300, respectively), incorporated by reference herein in their entireties. Briefly, rearranged human germline light chain targeting vector was made using VELOCIGENE® technology (see, e.g., U.S. Pat. No. 6,586,251 and Valenzuela et al. (2003) High-throughput engineering of the mouse genome coupled with high-resolution expression analysis, Nature Biotech. 21(6): 652-659) to modify mouse genomic Bacterial Artificial Chromosome (BAC) clones, and genomic constructs were engineered to contain a single rearranged human germline light chain region and inserted into an endogenous κ light chain locus that was previously modifie...
example 2
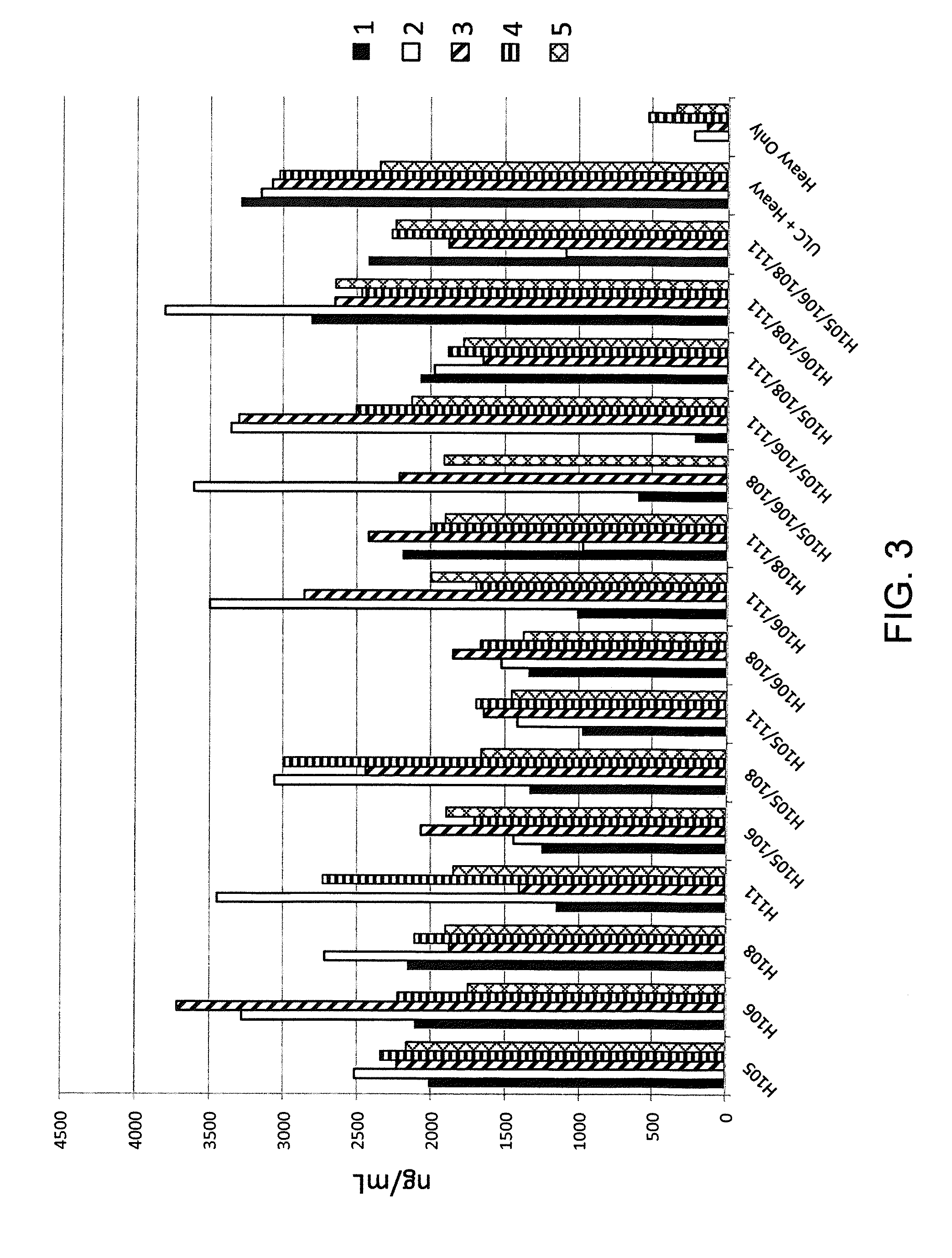
Engineering and Characterization of Histidine-Substituted Human Universal Light Chain Antibodies
example 2.1
Engineering of Histidine Residues into a Germline Human Rearranged Light Chain
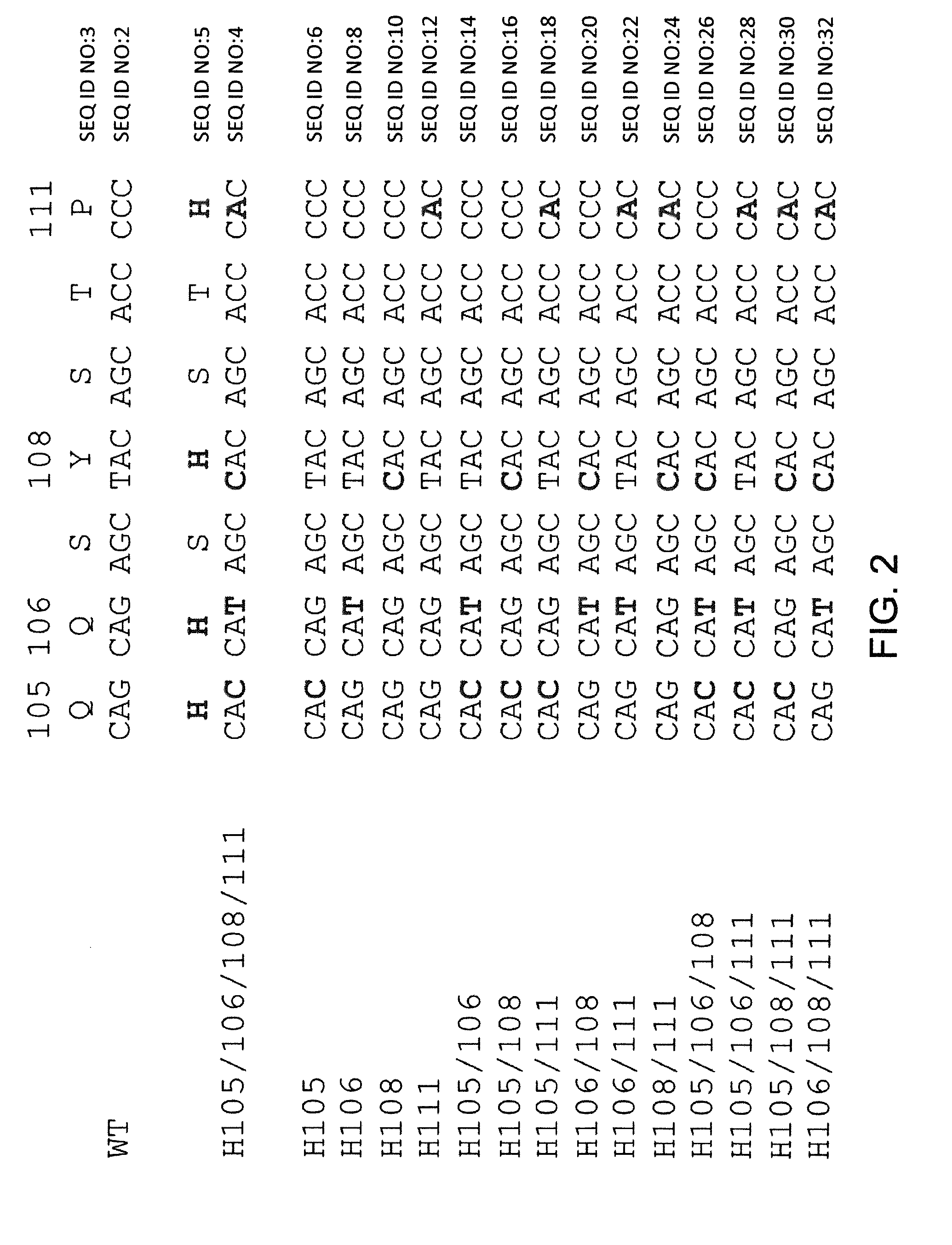
[0262]Histidine residues were engineered into a rearranged human Vκ1-39Jκ5 light chain using site directed mutagenesis primers specifically designed to introduce engineered histidine residues at Q105, Q106, Y108, and P111 positions of the human Vκ1-39Jκ5 light chain. Site directed mutagenesis was performed using molecular techniques known in the art (e.g., QuikChange II XL Site Directed Mutagenesis Kit, Agilent Technologies). Locations of the engineered residues in the CDR3 are shown in FIG. 2, the nucleic acid sequences of histidine-substituted CDR3's depicted in FIG. 2 are set forth in SEQ ID NOs: 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24, 26, 28, 30, and 32 (corresponding amino acid sequences are set forth in SEQ ID NOs: 5, 7, 9, 11, 13, 15, 17, 19, 21, 23, 25, 27, 29, 31, and 33). The nucleic acid and amino acid sequences of germline rearranged Vκ1-39Jκ5 CDR3 are set forth in SEQ ID NOs: 2 and 3, respect...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com