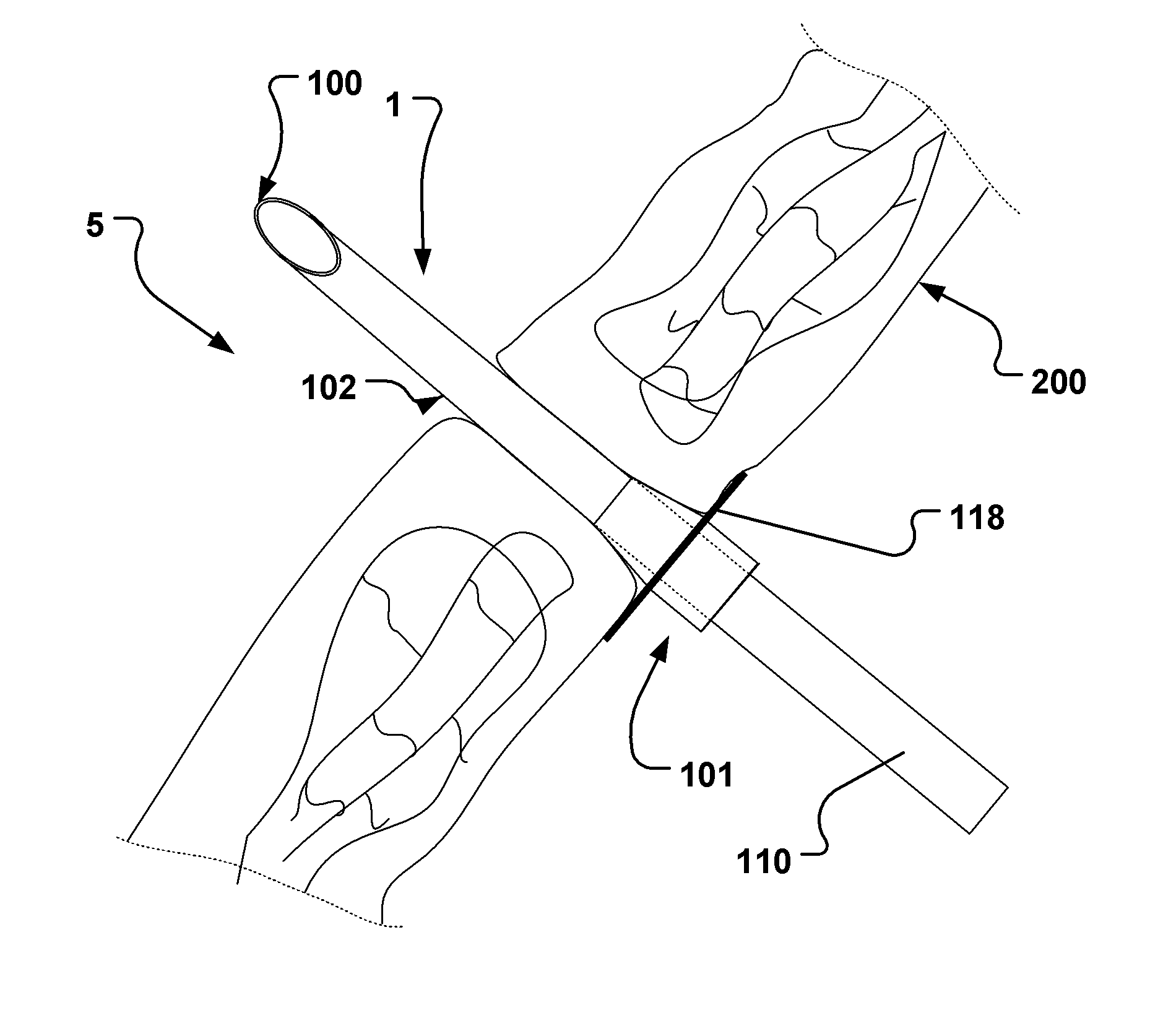
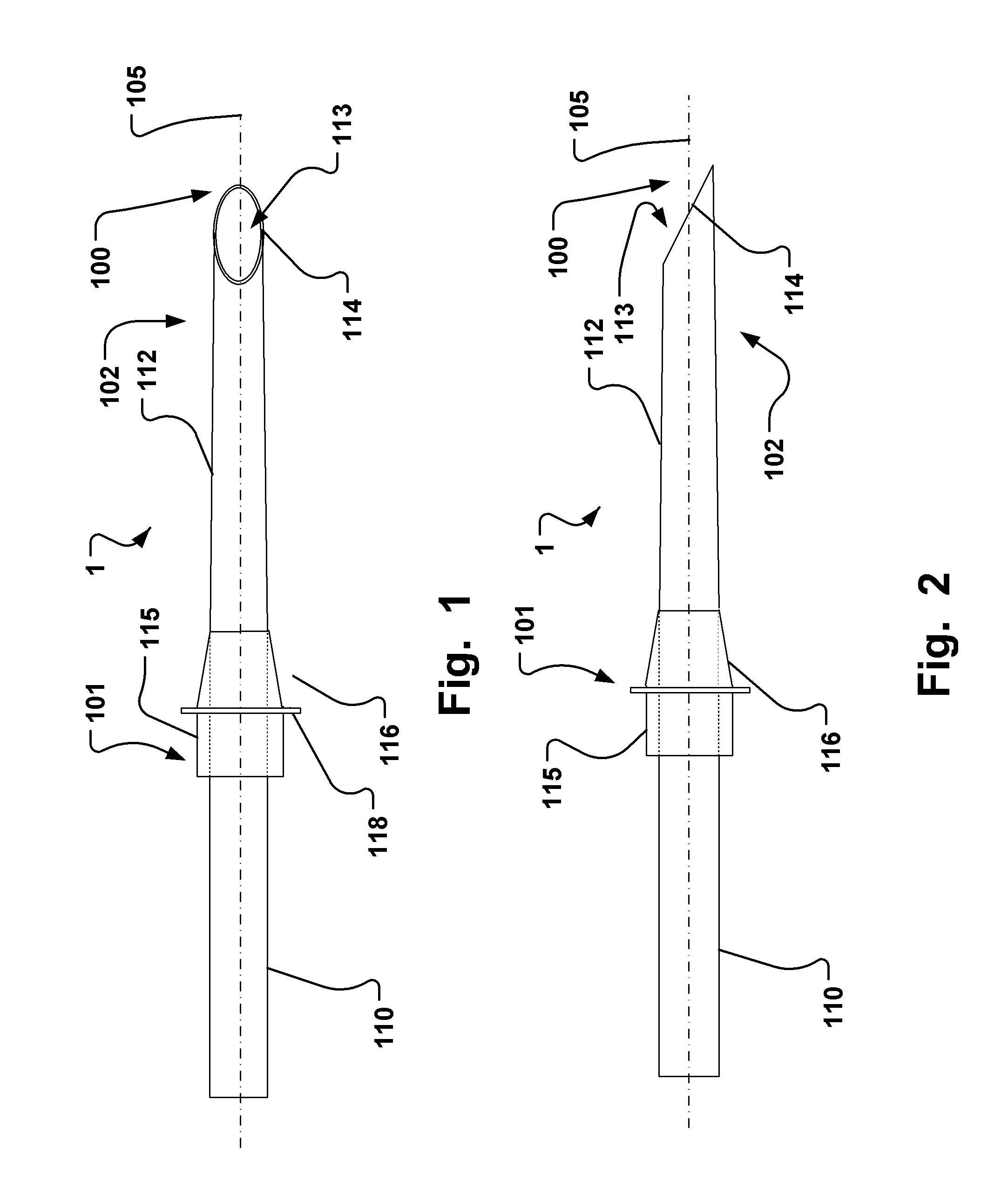
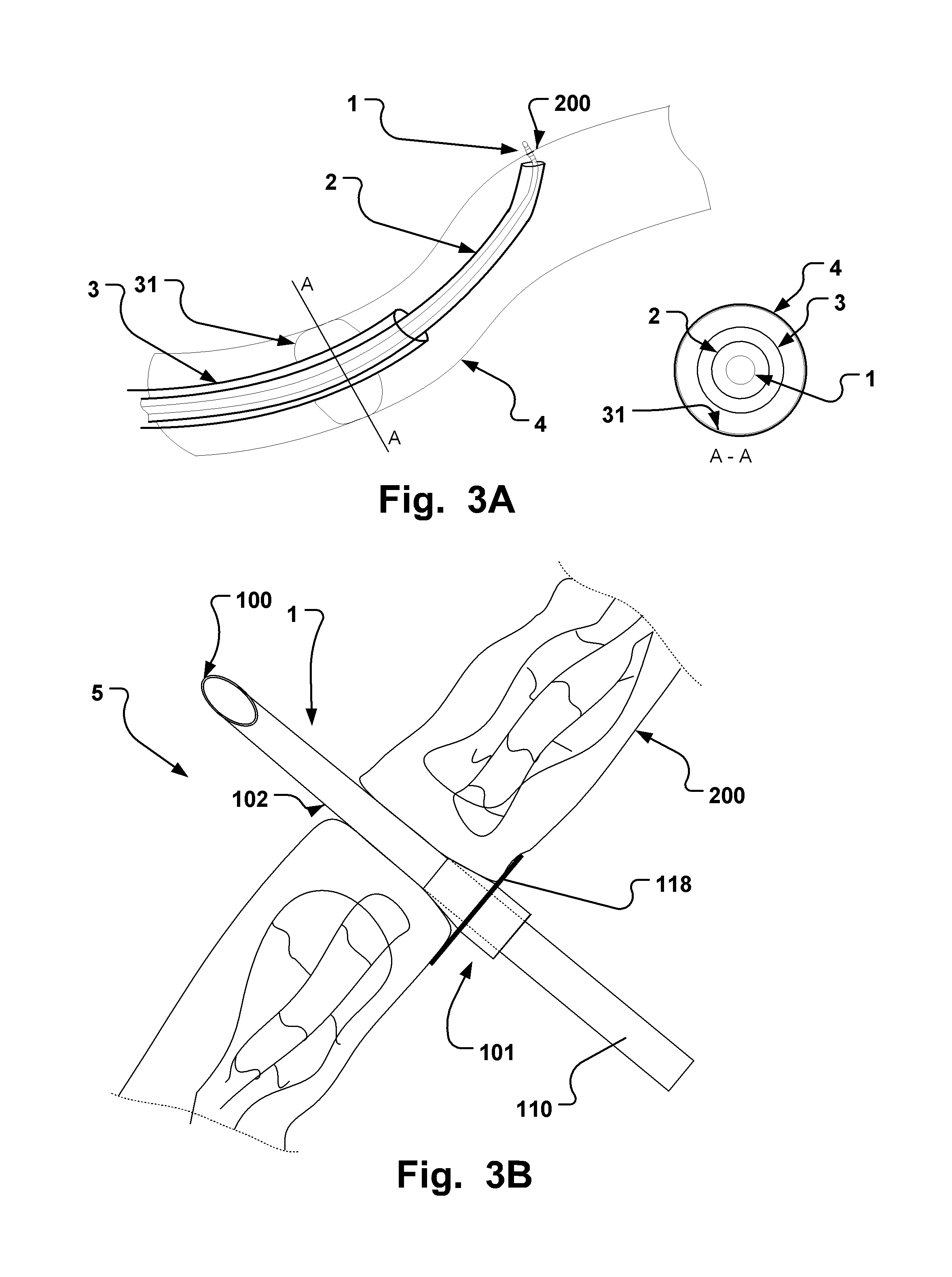
Endoluminal medical access device
a medical access device and endoluminal technology, applied in the field of catheter based medical devices, can solve the problems of significant surgical risks, inaccessible target areas in the body, and inapplicable techniques for all organ systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0142]In Table 1, given below, typical dimensions of some embodiments are given.
TABLE 1Typical dimension ranges and ratios of some embodiments of the extroducerdimensions in micrometers,Length ofapproximalLength ofLength ofhollowmeasuresdistal penetrationdetachmentLumenFlangeonlyportion 102limiterzoneOuter diameterdiameterdiameterMinApprox. 3xa few0, if the140100300outer diametermicrometerscatheter isof extroducer10-20dissolved400-450no zone isneededMaxNo limit if itMust notLimited byLimited by polymer Limited byLimited byis made of aaffect bloodtime / energytube and micro polymerdimensionshighly flexiblestream.amount ofcatheter system, tube andof the vessel.material,Approx 0.3dissolvedand intended microThe wheelsuch astimes vesselmaterial.target sitecatheterdesign isnitinol. Itdiameter,Processsystem andlimited bymust betypicallymust notintendedthe vesselpossible to250 harm tissue.target sitediameter toadvance itmicrometerSmallerapprox.through theis better.1.5x ofvascular treeApprox. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com