Alkylamine functionalized metal-organic frameworks for composite gas separations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
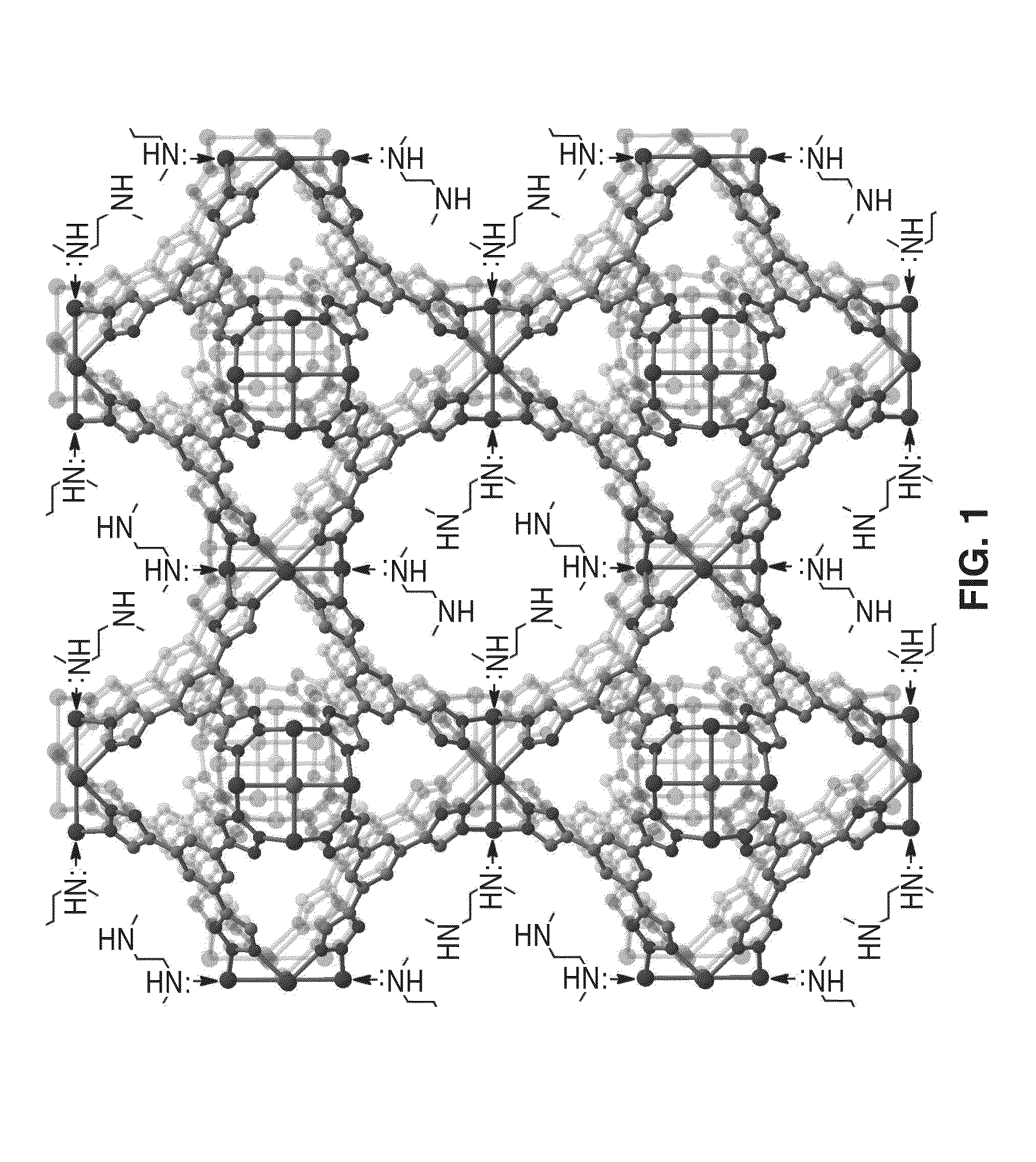
[0070]In order to demonstrate the functionality of metal-organic frameworks featuring coordinatively-unsaturated metal centers for separating gases, a mmen-CuBTTri framework as shown in FIG. 1 was constructed and tested.
[0071]A sample of CuBTTri (100.0 mg, 32.4 / μmol) was suspended in 10 mL of anhydrous hexane under nitrogen, and 75.4 μL (61.2 μg, 701 μmol, 1.8 equivalents per unsaturated CUII site) of N,N′ dimethylethylenediamine (mmen) was added via micropipette with stirring. The compound immediately turned blue and the suspension was heated at reflux for 18 hours under nitrogen. The solid was collected by filtration and washed with successive aliquots of hexane (5×10 mL) to remove unreacted diamine. The solid was then dried under reduced pressure to remove hexane. Anal. Calcd. for C144H195Cl3Cu12N96 (Mw=4139.6 g / mol): C, 41.78; H, 4.75; N, 32.48. Found: C, 42.23; H, 4.47; N, 32.05.
[0072]The grafted material, mmen-CuBTTri, was then activated by heating at 50° C. for 24 hours under...
example 2
[0074]In order to demonstrate the functionality of metal-organic frameworks featuring coordinatively-unsaturated metal centers for separating gases, a mmen-CuBTTri framework as shown in FIG. 1 was constructed and tested.
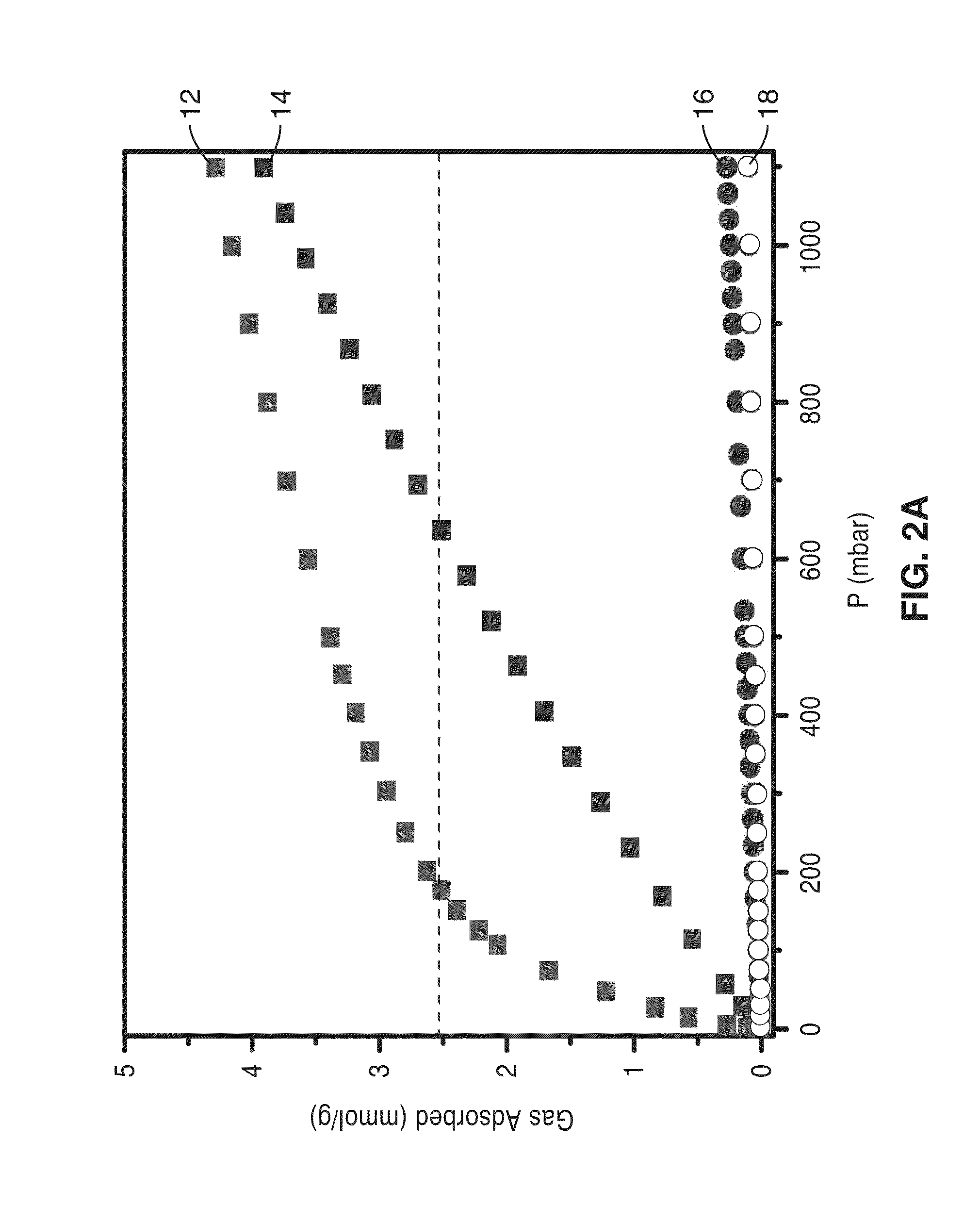
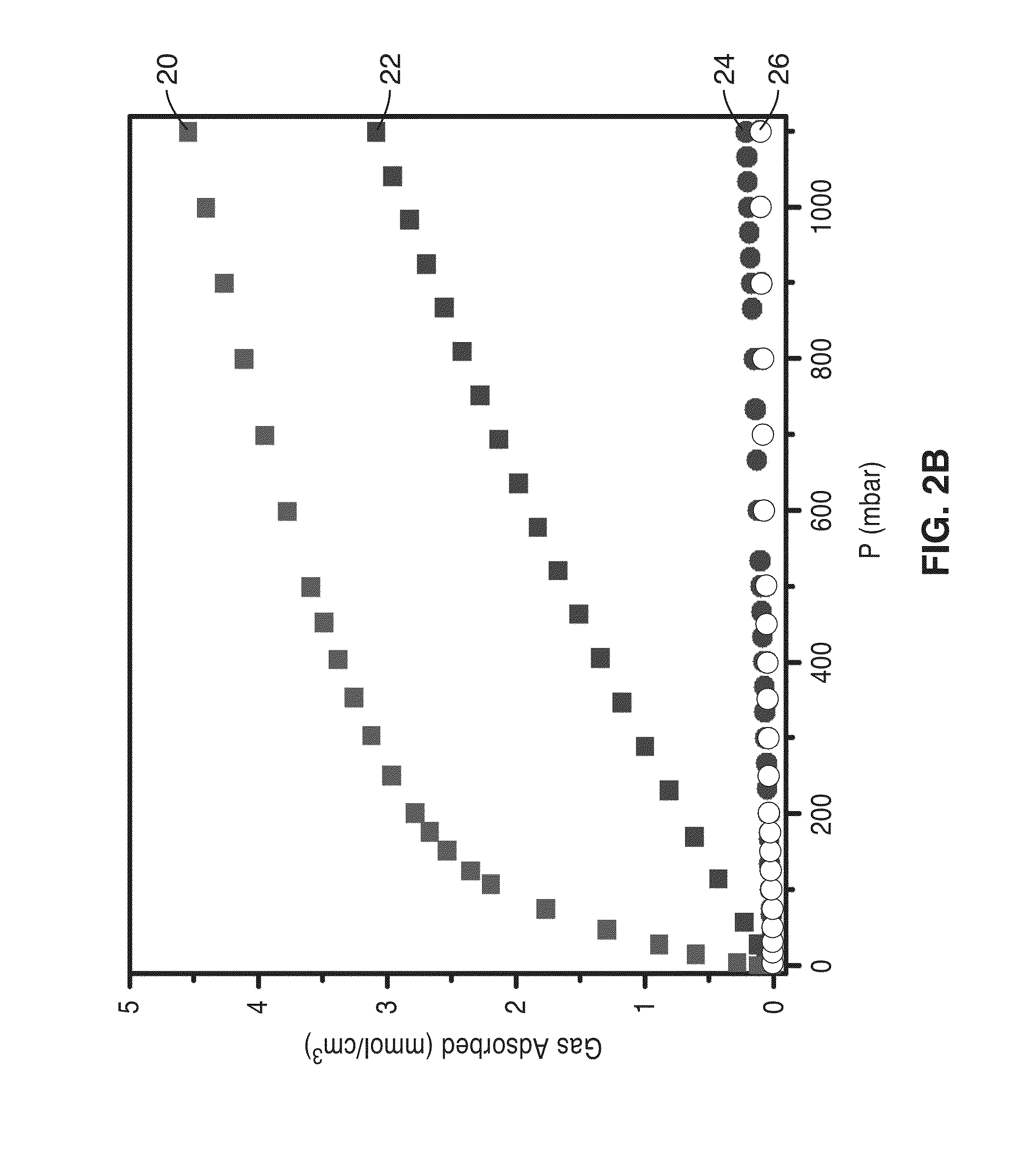
[0075]The mmen-CuBTTri and men-CuBTTri adsorbents were initially tested in the context of separating a mixture stream including CO2 and N2 to obtain a stream richer in N2 as compared to the mixture stream, with the adsorption of CO2 in the frameworks at low pressures.
[0076]Gas adsorption isotherms for pressures in the range 0-1.1 bar were measured by a volumetric method using a Micromeritics ASAP2020 instrument. A sample was transferred in an N2 filled glovebag to a pre-weighed analysis tube, which was capped with a transeal and evacuated by heating (50° C. for mmen-CuBTTri, 100° C. for men-CuBTTri) under dynamic vacuum for 24 hours. The evacuated analysis tube containing the degassed sample was then carefully transferred to an electronic balance and weighed again to...
example 3
[0088]To further characterize the mmen-CuBTTri frameworks, the isosteric heat of adsorption and working capacity of the materials were analyzed. Utilizing a dual-site Langmuir adsorption model, isosteric heats of adsorption were calculated for CO2 in mmen-CuBTTri and compared to those obtained from data for bare CuBTTri, which were fit using a single-site Langmuir model to give a value of −24 kJ / mol. The N2 adsorption isotherm for mmen-CuBTTri was also fit to a single-site Langmuir model, resulting in a calculated isosteric heat of adsorption of −15 kJ / mol.
[0089]The isosteric heat of CO2 adsorption in mmen-CuBTTri approaches −96 kJ / mol at zero coverage, corresponding to the largest value yet reported for CO2 adsorption in a metal-organic framework. For comparison to mmen-CuBTTri, the heat of adsorption for en-CuBTTri was recalculated with the same dual-site Langmuir model. Because this model incorporates absolute adsorption, direct comparisons between the two different models are no...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Partial pressure | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
| Heat | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com