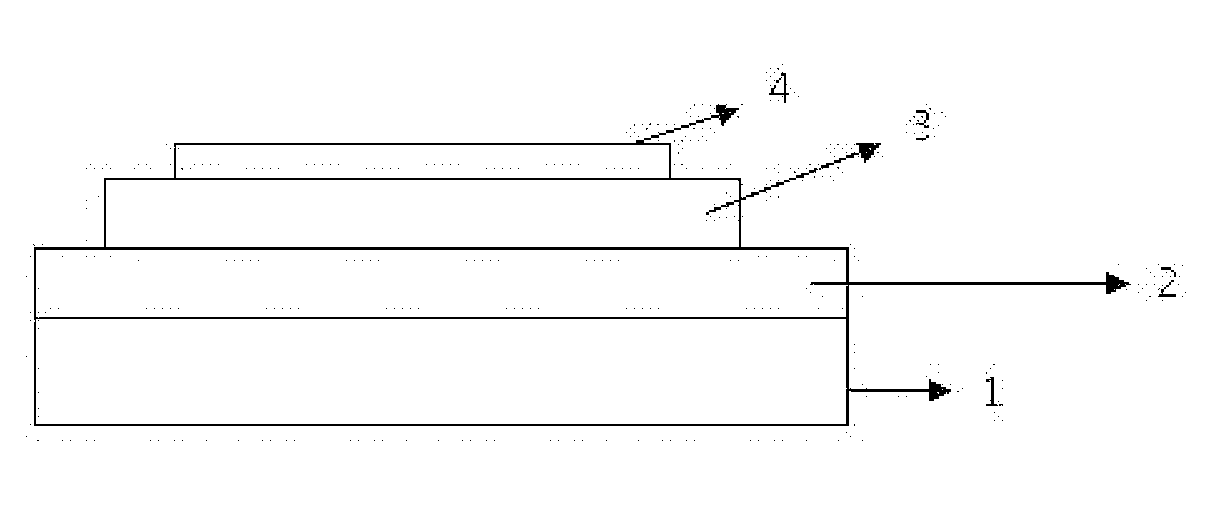
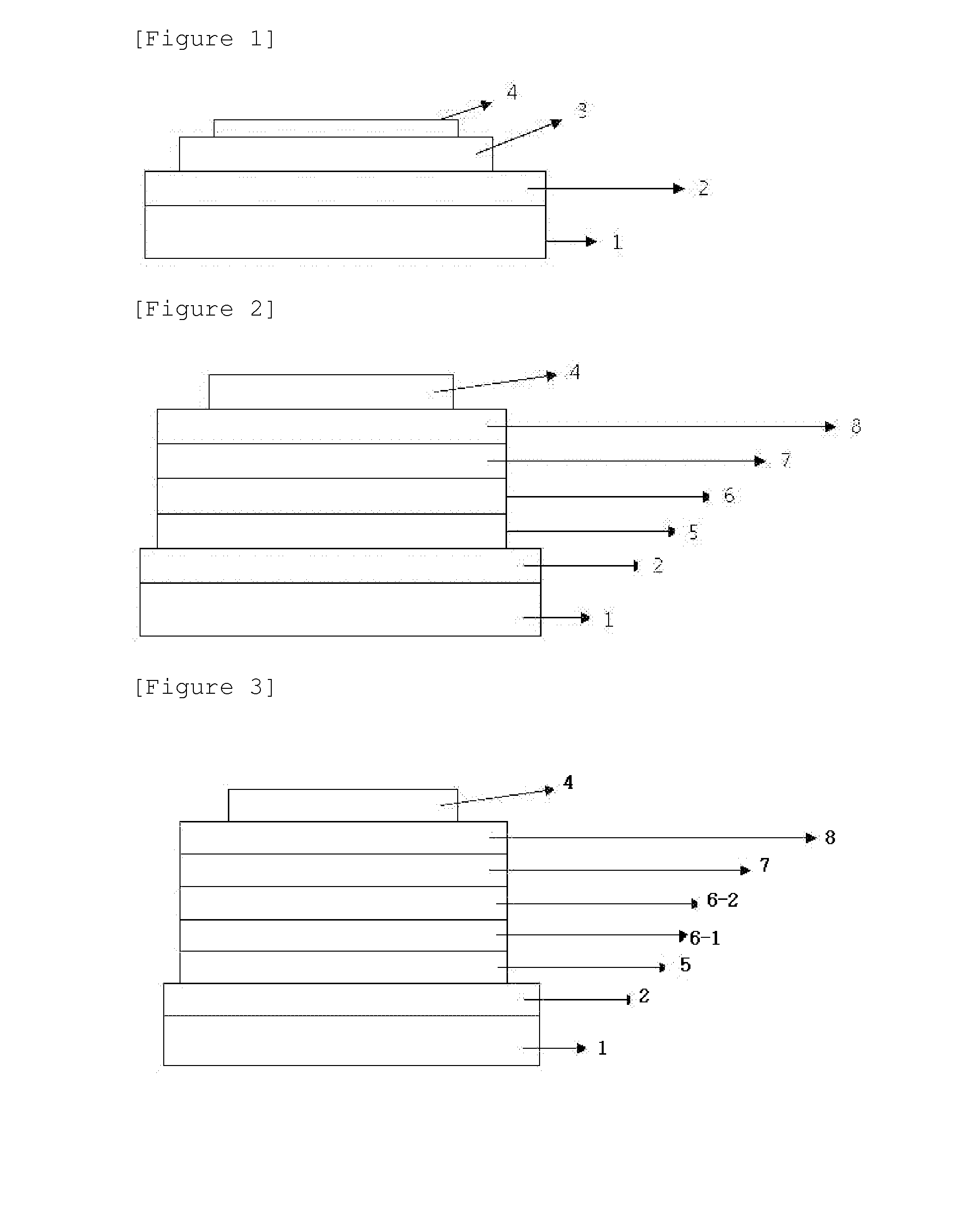
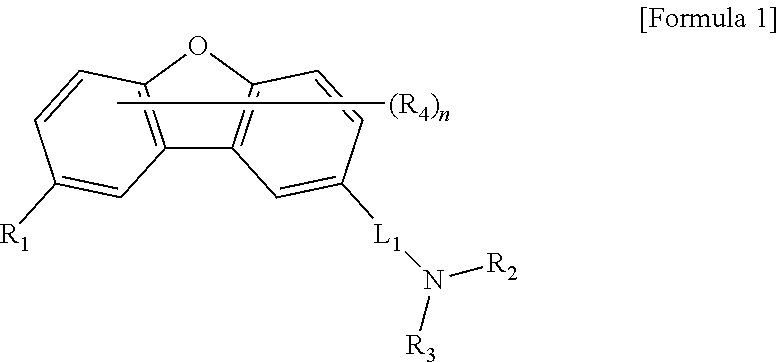
Material for organic light-emitting device, and organic light-emitting device using same
a technology of light-emitting devices and materials, which is applied in thermoelectric devices, organic chemistry, triarylamine dyes, etc., can solve the problems of difficult preparation of organic light-emitting devices having high efficiency and a long service life, and requiring high electric currents, so as to improve the service life characteristic of the device, reduce the driving voltage of the device, and improve the effect of light efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthetic example 1
Preparation of Compound Represented by Formula 1-1
[0110]
(1) Preparation of Formula 1A
[0111]2-bromodibenzofuran (30 g, 121 mmol), 4-chlorophenyl boric acid (20.9 g, 134 mmol) and potassium carbonate (K2CO3) (39.4 g, 285 mmol) were dissolved in tetrahydrofuran (THF) (300 ml) and H2O (100 ml) and the solution was heated at 50° C. Tetrakis(triphenylphosphine) palladium (Pd(PPh3)4) (2.8 g, 2.42 mmol) was added thereto and then the mixture was refluxed for 12 hours. The mixture was cooled to normal temperature, and then the water layer was removed. Magnesium sulfate (MgSO4) was put into the organic layer and then the mixture was filtered. The mixture was concentrated, and then was purified by column chromatography to obtain the compound of Formula 1A (25 g, yield 74%).
[0112]MS: [M+H]+=278
(2) Preparation of Formula 1-1
[0113]The compound of Formula 1A (10 g, 36 mmol), bisbiphenyl amine (11.6 g, 36 mmol), NaOtBu (4.2 g, 44.1 mmol) and xylene (100 ml) were mixed, and then the mixture was heat...
synthetic example 2
Preparation of Compound Represented by Formula 1-2
[0115]
(1) Preparation of Formula 1B
[0116]A compound of Formula 1B (30 g, yield 70%) was obtained in the same manner as in the preparation of the compound 1A in Synthetic Example 1, except that a compound 4-chlorobiphenyl boronic acid (32 g, 134 mmol) was used instead of the compound 4-chlorophenyl boronic acid.
[0117]MS: [M+H]+=354
(2) Preparation of Formula 1-2
[0118]The compound of Formula 1B (10 g, 28.2 mmol), bisbiphenyl amine (9.1 g, 28.2 mmol), NaOtBu (3.4 g, 35.1 mmol) and xylene (100 ml) were mixed and then the mixture was heated at 100° C. Bis[(tri-tertiary-butyl)phosphine]palladium (Pd(p-t-Bu3)2) (138 mg, 0.27 mmol) was added thereto, and then the mixture was refluxed for 48 hours. The mixture was cooled to normal temperature, and then was purified by column chromatography. The mixture was dried to obtain the compound of Formula 1-2 (15 g, yield 83%).
[0119]MS: [M+H]+=639
synthetic example 3
Preparation of Compound Represented by Formula 1-3
[0120]
(1) Preparation of Formula 1C
[0121]The compound 1A (30 g, 102 mmol) was introduced into a flask including 1 L of dichloromethane to dissolve the compound 1A, then a solution in which bromine (5.30 ml, 108 mmol) was diluted with 400 ml of dichloromethane was slowly added dropwise to the flask, and the mixture was stirred for 12 hours. After the reaction was completed, the reactant solution contained in the flask was washed with a sodium hydrogen carbonate saturated aqueous solution, then the organic layer was separated from the flask, dried over anhydrous magnesium sulfate and then filtered. The filtrate was concentrated and then recrystallized with dichloromethane and ethanol to obtain a white solid compound (15 g, 39%).
[0122]The compound along with phenyl boronic acid (5.63 g, 46.2 mmol) and potassium carbonate (K2CO3) (19.1 g, 138 mmol) was dissolved in tetrahydrofuran (THF) (400 ml) and water (150 ml) and the solution was he...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com