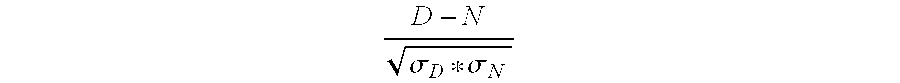Glomerulonephritis biomarkers
a glomerulonephritis and biomarker technology, applied in the field of glomerulonephritis biomarkers, can solve the problems of increased risk of gn, blood and protein loss in urine, and difficulty in determining gn,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Measurement of biomarkers indicative of Glomerulonephritis
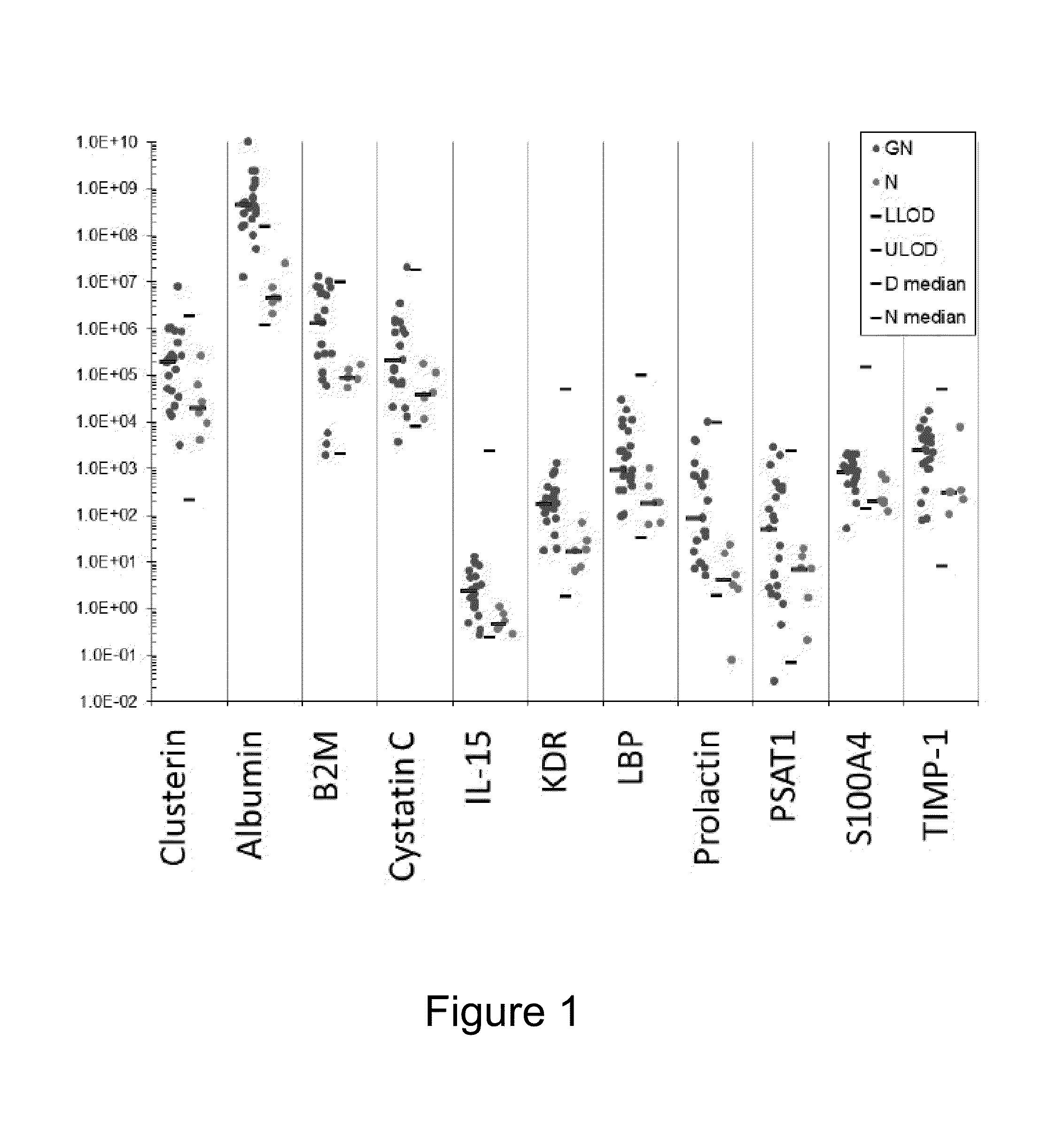
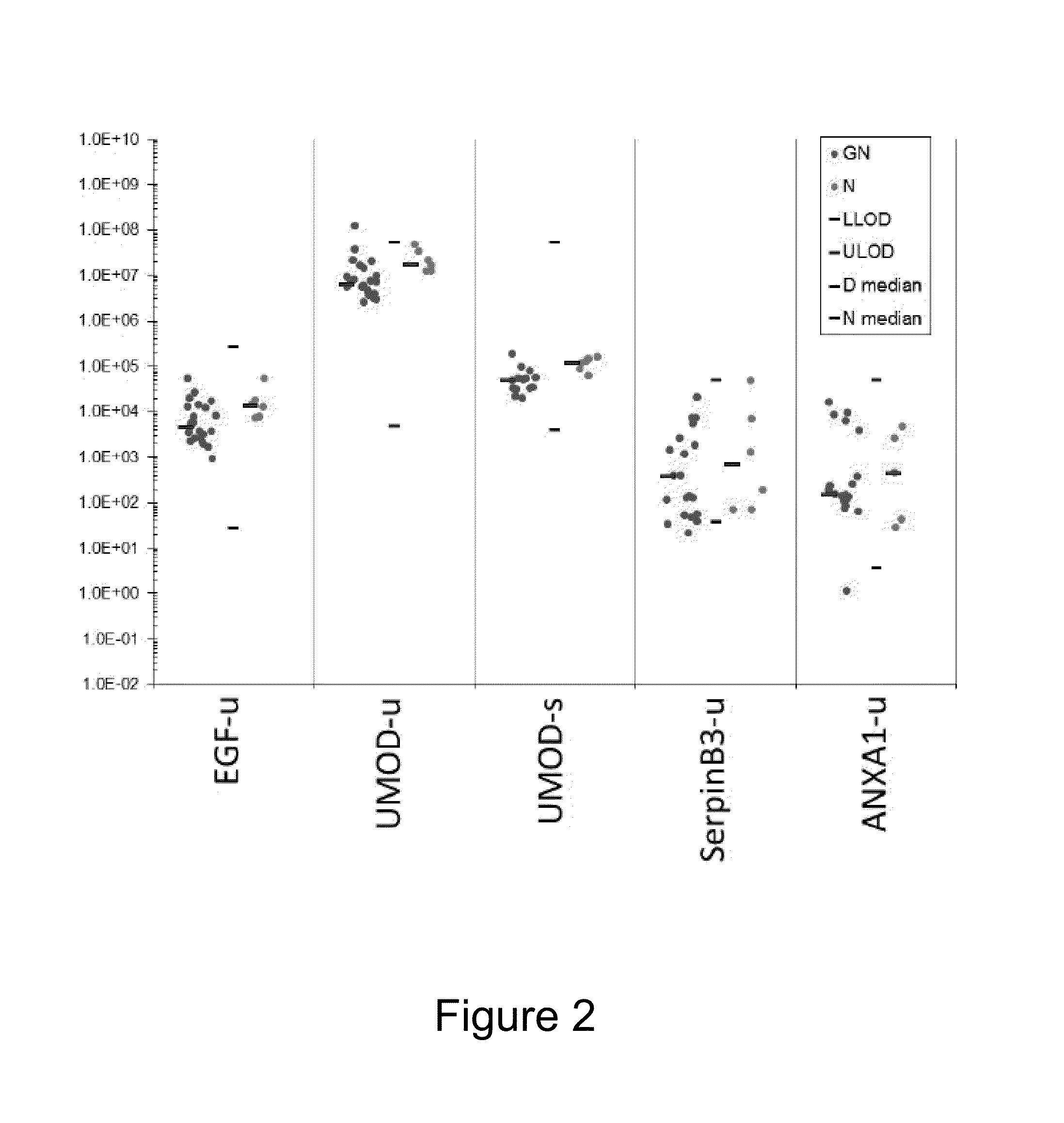
[0091]Urine and serum samples were collected from 23 patients previously diagnosed with GN and 6 normal control subjects (patients having normal kidney function). Samples were analyzed in an immunoassay format and the samples were screened for the presence / absence of the following set of biomarkers: AKR1B1, AKR1C2, Albumin, ALP, ANXA1, B2M, BCL2L2, Calbindin, CHGA, Clusterin, CRYAB, Cystatin C, E-Cadherin, EGF, EOTAXIN, EOTAXIN-3, FABP5, GCLM, GLO1, GM-CSF, GPI, GSTM1, GSTM2, ICAM-1, IFN-gamma, IFN-gamma, IL-10, IL-12P70, IL-13, IL-15, IL-16, IL18, IL-1 alpha, IL-1 beta, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IP-10, KDR, KIM-1, LBP, MAGEA4, MAPK14, MBD1, MCP-1, MCP-4, MDC, MIP-1B, NGAL, NME2, OCL, ODC1, ONN, OPGN, OPN, osteoactivin, Osteopontin, P-Cadherin, PPP2R4, PRDX4, Prolactin, PSAT1, RAC1, RANTES, S100A4, S100A6, SAT1, SERPINB3, SFN, SOST, TARC, TFF3, TIMP-1, TNF RI, TNF RII, TNF-alpha, UMOD, VCAM-1, VEGF and aGST.
[0092]In...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com