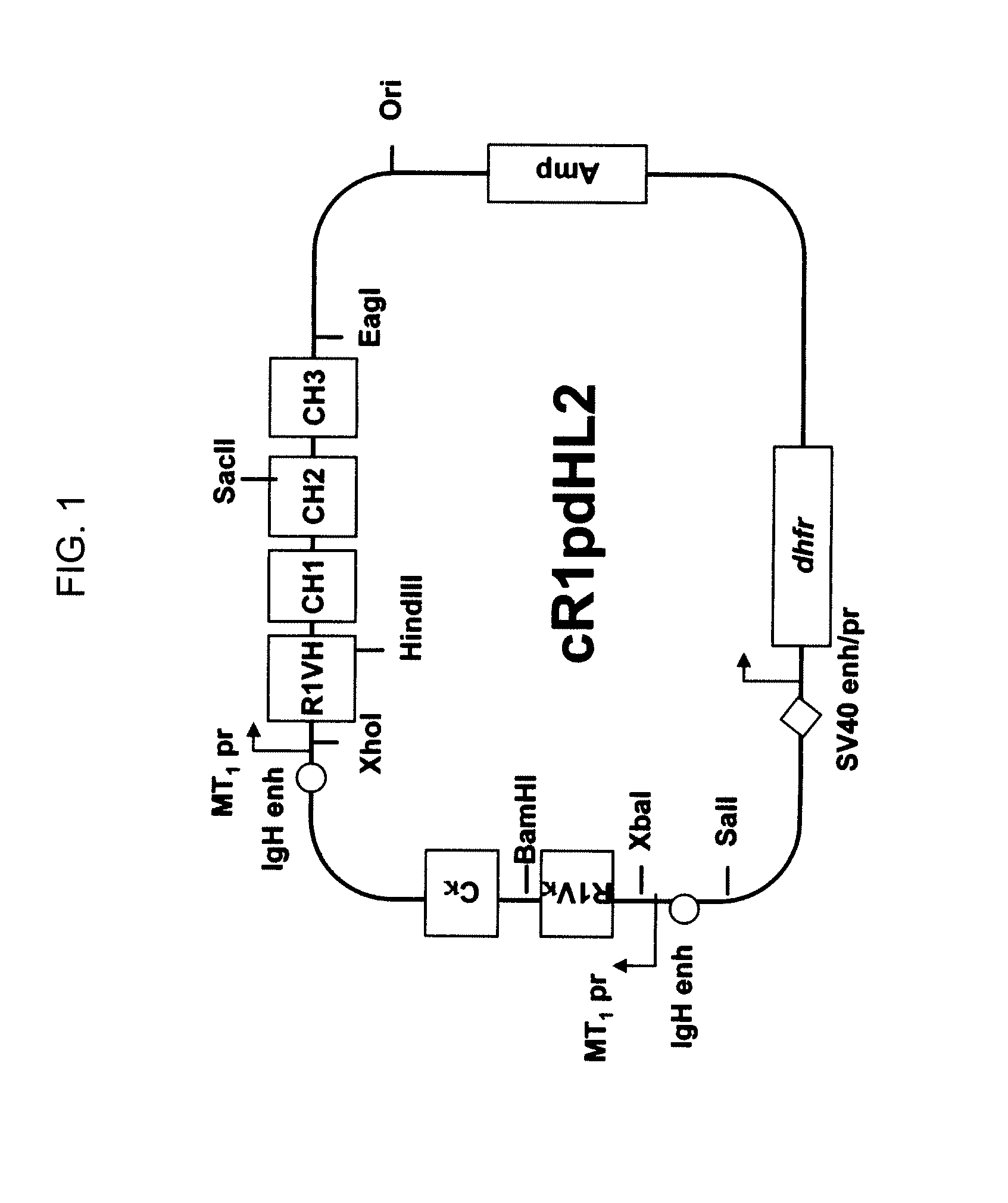
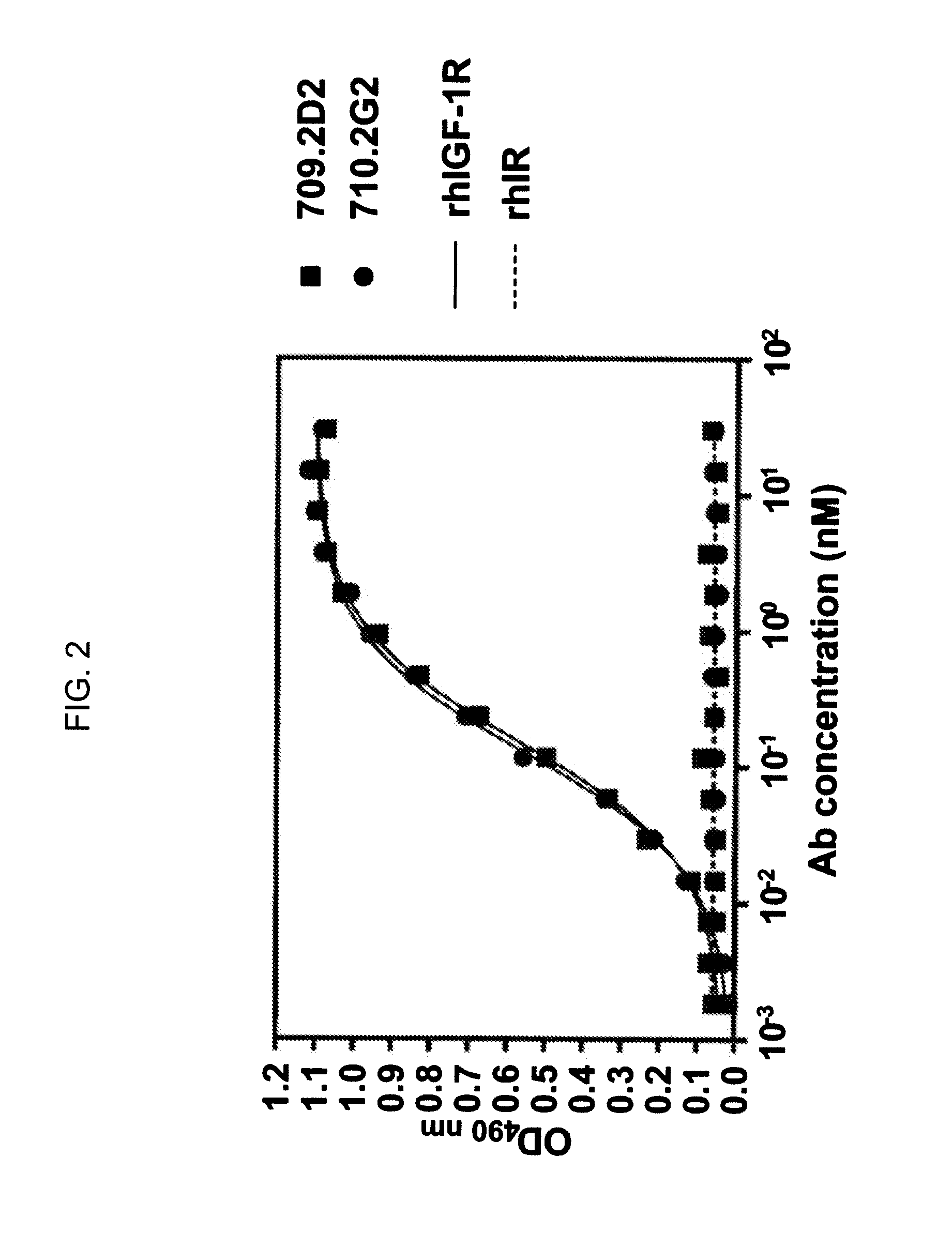
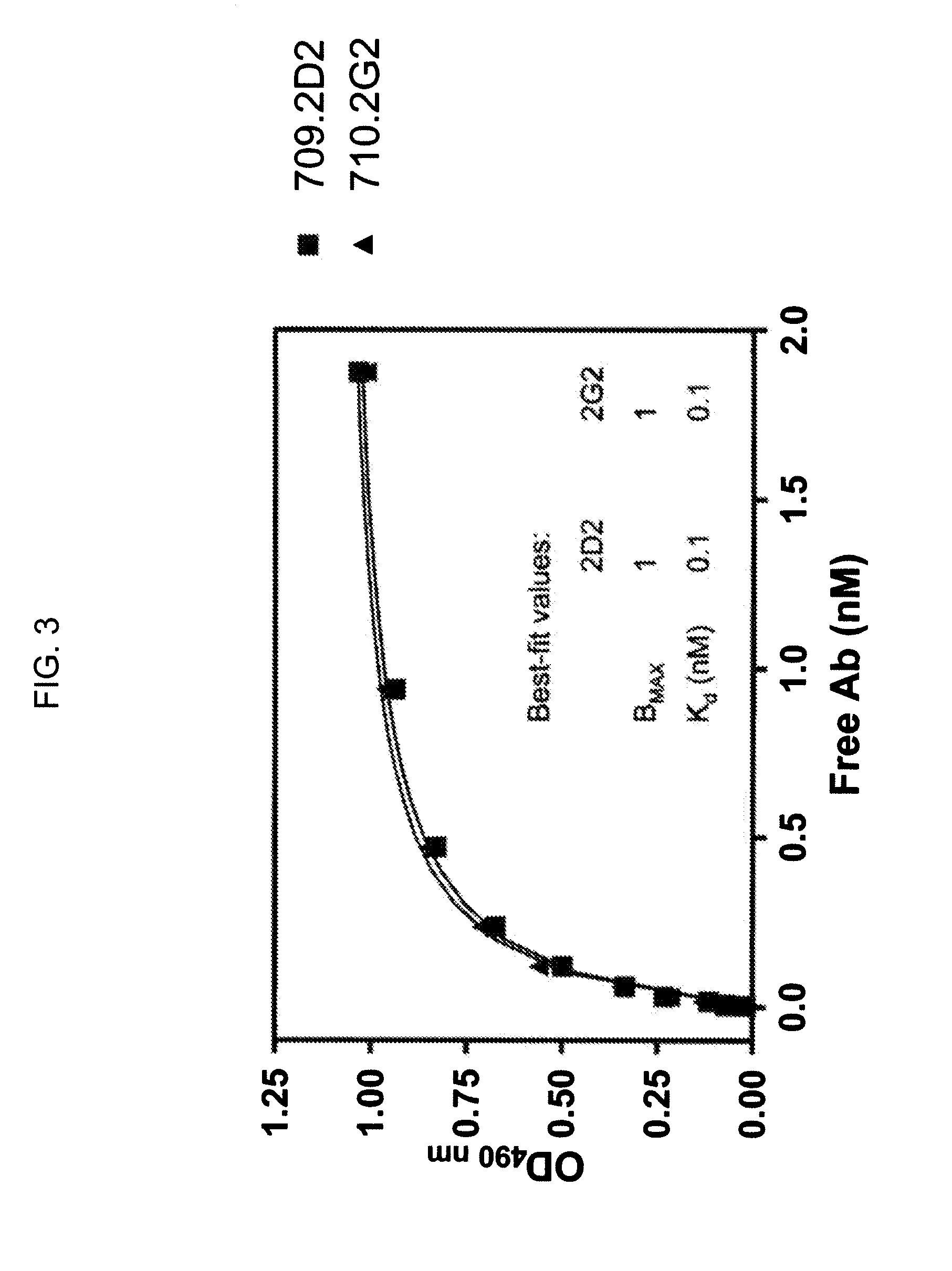
Novel Class of Monospecific and Bispecific Humanized Antibodies that Target the Insulin-like Growth Factor Type I Receptor (IGF-1R)
a technology of insulinlike growth factor and humanized antibodies, which is applied in the field of monospecific and bispecific humanized antibodies that target the insulinlike growth factor type i receptor (igf1r), can solve the problems of limited use of such agents in cancer therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation and Initial Characterization of Anti-IGF-1R Antibodies: R1, cR1, and hR1
[0181]Three BALB / c mice were each immunized i.p. with 15 μg of recombinant human IGF-1R (R&D Systems, Catalog #391-GR), comprising a mixture of both processed and unprocessed extracellular domain of human IGF-1R, in complete Freund's adjuvant. Additional immunizations in incomplete Freund's adjuvant were done 14, 21, and 28 days after the initial immunization. Spleen cells from the immunized mice were fused with P3X63Ag8.653 cells to generate hybridomas according to standard protocols. One clone (C-11) expressing anti-IGF-1R but not anti-IR (insulin receptor) activity was isolated and expanded in cultures to obtain the mouse antibody designated ML04R1 or R1, which was shown to be an IgG1 / k with the ability to inhibit the binding of radioiodinated IGF-1 to the IGF-1R expressing human breast cancer cell line MCF-7L (a subline of MCF-7) comparable to a commercially available mouse anti-IGF-1R monoclonal ...
example 2
Epitope Mapping Studies of R1, cR1, and hR1
[0186]To further locate the region of IGF-1R to which hR1 binds, a panel of commercially available anti-IGF-1R mAbs that have their epitopes to IGF-1R mapped, were evaluated for their ability to cross-block each other from binding to the IGF-1R-coated beads. The results of two typical experiments are provided in FIG. 8A, which shows the binding of R1 tagged with a fluorescent probe (PE) was not affected by MAB391 even at 100 μg / mL, and FIG. 8B, which shows the binding of MAB391 tagged with PE was only partially inhibited (50 to 60%) by R1 at 100 μg / mL. Additional results summarized in Table 3A indicate that the epitope of R1 is located in the CR domain between aa 151 and 282 and can be further located to the first half of the CR domain between aa 151 and 222 (Table 3B).
TABLE 3A% binding of each labeled antibody (*) to rhIGF-1R-coated beads in thepresence of the unlabeled antibody (24-31, 24-57, 17-69, 1-2, 1H7, 2C8, 3B7)Anti-IGF-1R24-3124-5...
example 3
Additional characterization of R1, cR1, and hR1
[0187]Whereas IGF-1 stimulates proliferation of MCF-7 cells grown in serum-free medium, achieving a maximal effect of 50% increase in viable cell counts at 100 ng / mL when compared to the untreated control at 48 h, hR1 does not (FIG. 9). Thus hR1 is not agonistic upon binding to IGF-1R. Internalization of hR1 into MCF-7 was observed at 37° C. but not at 4° C. (not shown).
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| decay-energy | aaaaa | aaaaa |
| decay-energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com