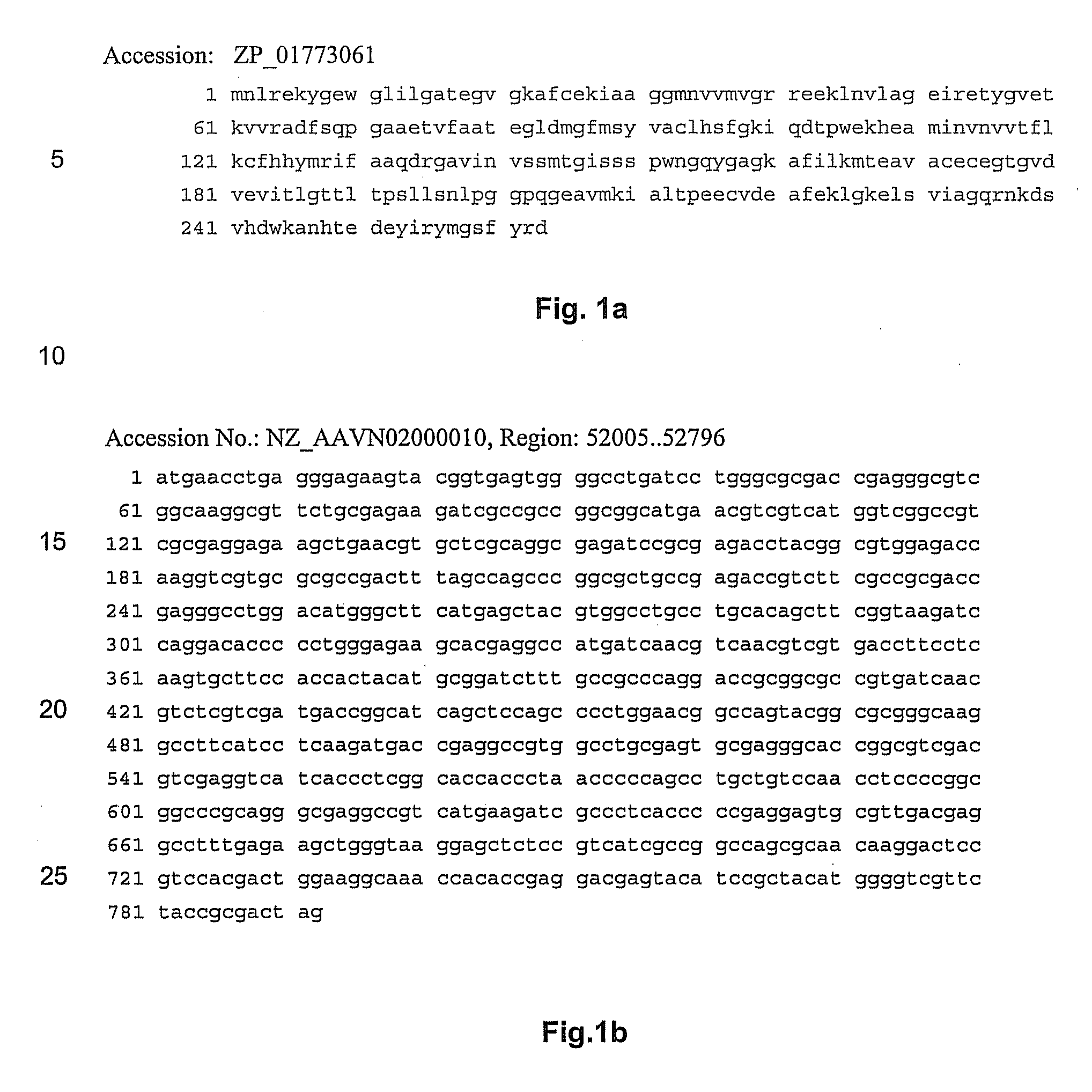
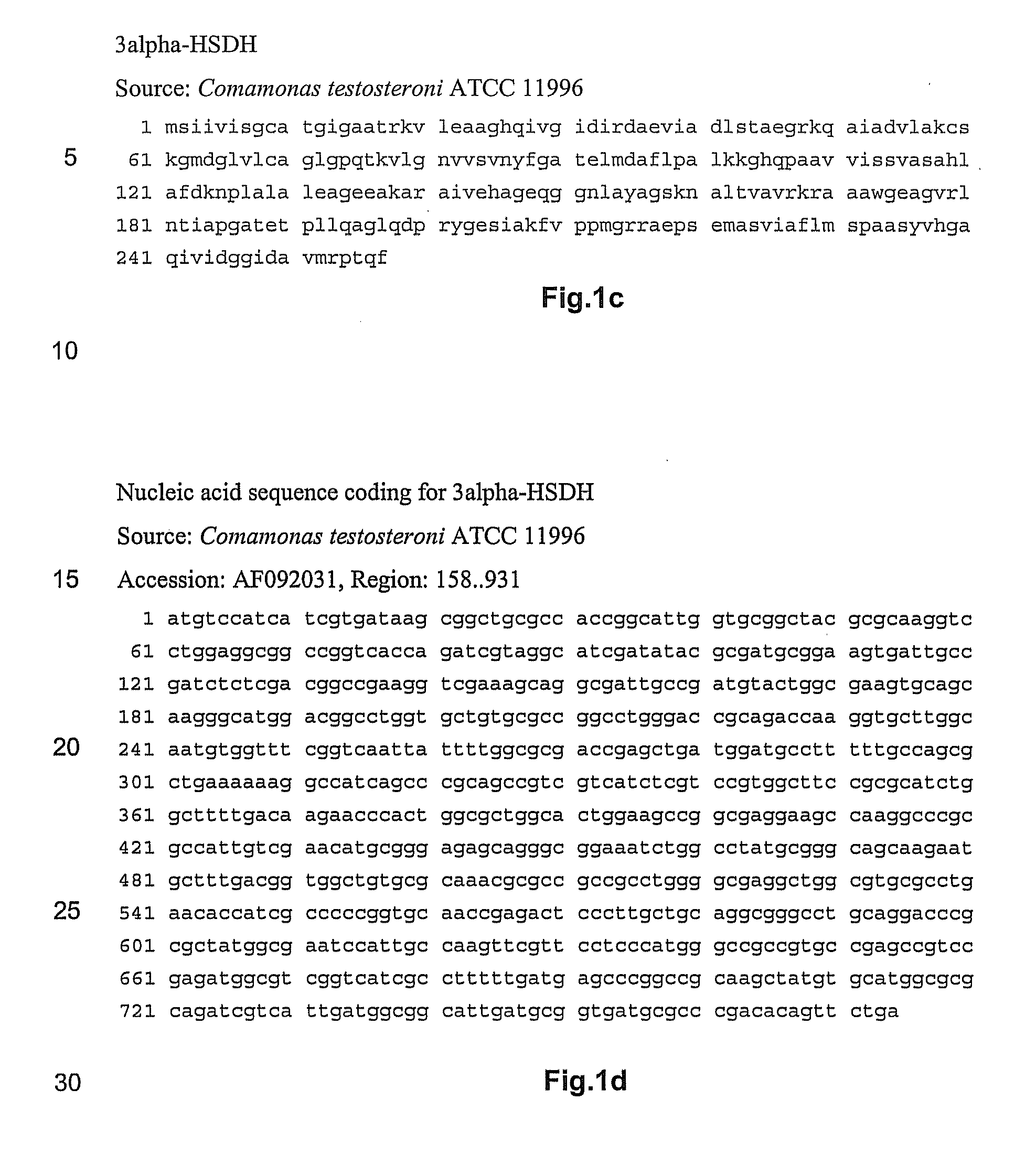
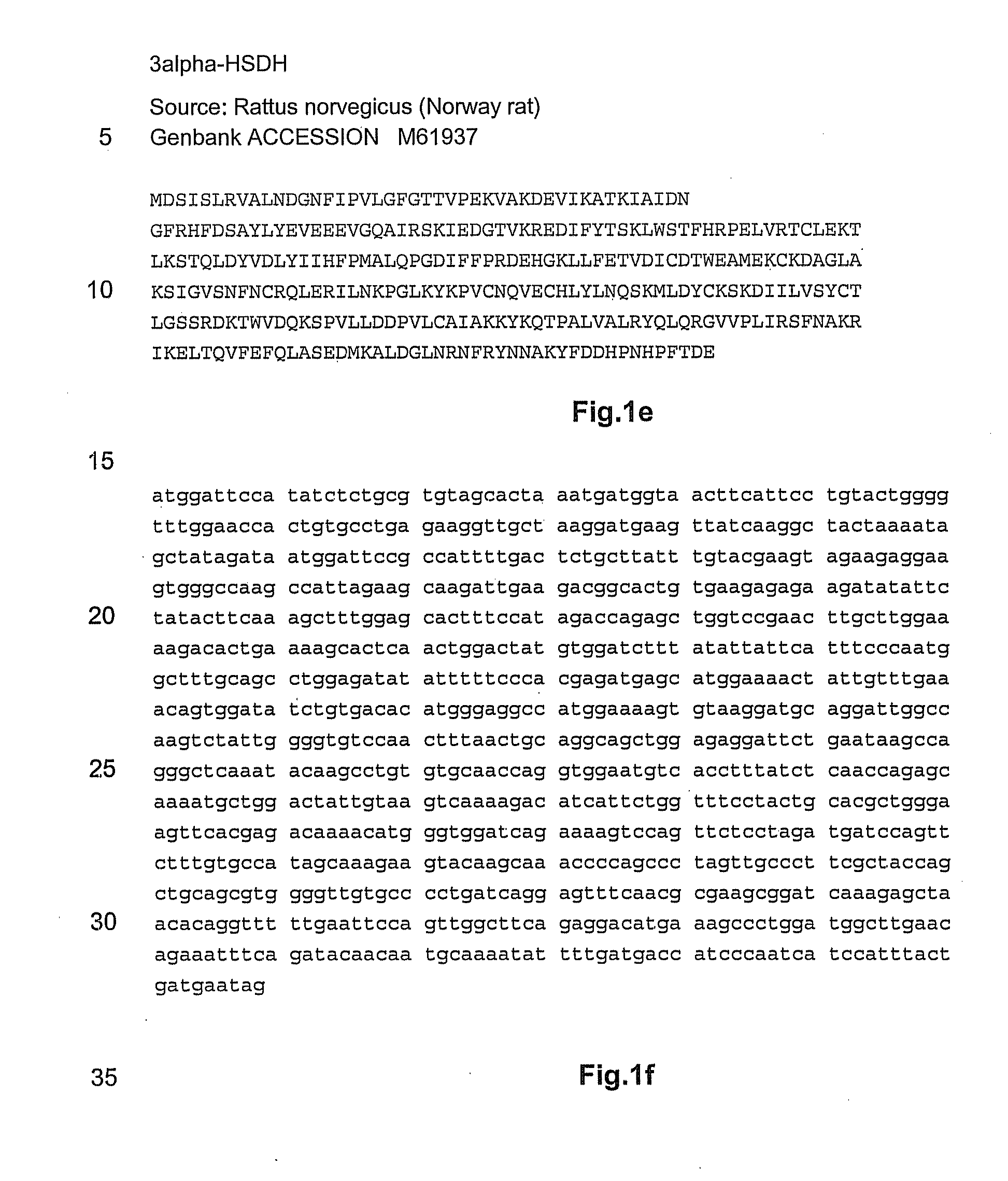
NOVEL 7beta-HYDROXYSTEROID DEHYDROGENASES AND THEIR USE
a technology of hydroxysteroid dehydrogenase and hydroxysteroid, which is applied in the direction of oxidoreductase, biochemistry apparatus and processes, enzymes, etc., can solve the problems of incomplete conversion of educt, increased process cost, and need for preparative purification of enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 4
5. An expression cassette, comprising a nucleic acid sequence as stated in embodiment 4 under the genetic control of at least one regulative nucleic acid sequence.
embodiment 5
6. A vector, comprising at least one expression cassette as stated in
7. A recombinant microorganism which bears at least one nucleic acid sequence as stated in embodiment 4 or at least one expression cassette as stated in embodiment 5 or is transformed with at least one vector as stated in embodiment 6.
8. A method for the production of a 7β-HSDH as stated in one of embodiments 1 to 3, wherein a microorganism as stated in embodiment 7 is cultured and the 7β-HSDH expressed is isolated from the culture.
9. A method for the enzymatic synthesis of 7β-hydroxysteroids, wherein the corresponding 7-ketosteroid is converted in the presence of a 7β-HSDH according to the definition in one of embodiments 1 to 4, and at least one reduction product formed is optionally isolated from the reaction system.
embodiment 9
10. The method as stated in embodiment 9, wherein the ketosteroid to be reduced is selected from dehydrocholic acid (DHCA),
7-keto-lithocholic acid (7-keto-LCA),
7,12-diketo-lithocholic acid (7,12-diketo-LCA) and
the derivatives thereof, such as in particular a salt, amide or alkyl ester of the acid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Atomic weight | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com