Modified Cell Lines for Increasing Lentiviral Titers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
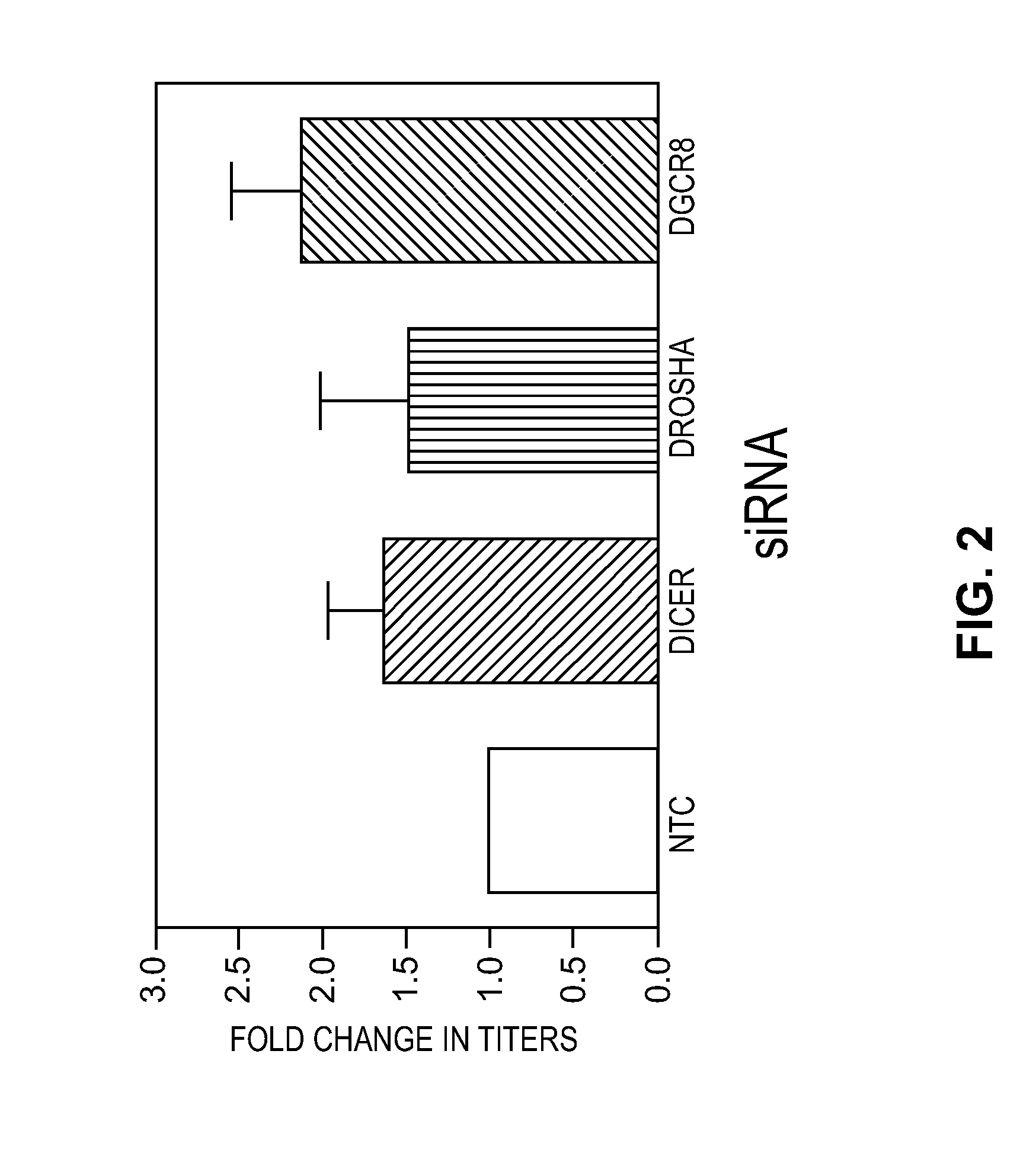
Effects of Knockdown of Genes Associated with the RNAi Pathway on Lentiviral Titer
[0030]To determine the effects of the RNAi pathway on lentiviral titer, HEK 293T cells (ATCC) were first seeded (6×105 cells / well, 6 well plate) and cultured for 18-24 hours. Subsequently, a pool of siRNAs (SMARTpools, Thermo Fisher Scientific, Dharmacon Products) targeting 1) Dicer (cat. no. M-003483-00-0005), 2) Drosha (cat. no. M-016996-02-0005), or 3) DGCR8 (cat. no. M-015713-01-0005) were transfected (25 nM) into the cells by lipid mediated transfection (DharmaFECT 1, Thermo Fisher Scientific, Dharmacon Products) using manufacturer's recommended procedures. The pools of siRNA were duplexes that contained sense strands that contained the sequences identified in Table I.
[0031]Twenty-four hours post-transfection (Day 3) cells were co-transfected with a pool of plasmids that included a lentiviral transfer vector (6 micrograms / well) and accessory packaging vectors (TransLenti Packaging Mix, Thermo Fish...
example ii
Effects of Knockdown of Hrs and Tetherin on Lentiviral Titers
[0035]Using the original protocol, the researchers investigated the effects that siRNA-mediated knockdown (KD) of Hrs and Tetherin (Thermo Fisher Scientific, Dharmacon Products, cat. no. M-011817-00-0005 (Tetherin) and M-016835-00-0005 (Hrs)) would have on lentiviral titers. As shown in FIG. 5, KD of Hrs provided only modest increases in viral titers (<1.5× effects). In contrast, KD of Tetherin induced significant increases in viral titer (greater than two fold, p<0.05).
example iii
Effects of Over-Expression of Annexin2 and Tsg101 on Lentiviral Titers
[0036]To test the effects that over-expression of Annexin2 has on lentiviral titers, lentiviral particles capable of expressing the Annexin2 or Tsg101 open reading frame (ORF) were transduced into HEK293T cells at an MOI of either 0.3 or 3.0 (LentiORF, Thermo Fisher Scientific, Open Biosystems Products cat. no. PLOHS—100003638 (Annexin 2) and PLOHS—100005422 (Tsg101)). Subsequently, cultures were grown in the presence of blasticidin (10 ug / ml, 2 week) to select for stable integrants. Cells were then expanded for 1 week in the absence of blasticidin, seeded at a density of 1.2×106 cells per well in a 6 well plate, and then transfected with a transfer vector / Trans-Lenti packaging mixture (available from Thermo Scientific).
[0037]The results of these studies are shown in FIG. 6 which shows that over-expression of TSG101, as well as Annexin 2 increase in viral titers.
[0038]The studies described above have identified mu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com