Cerebrospinal Fluid Purification System
a cerebrospinal fluid and purification system technology, applied in the field of medical devices and methods, can solve the problems of not disclosing the removal of csf, no csf purification system that allows direct, targeted, logical and disease-specific removal of one or more target compounds, and system has several inherent limitations, so as to achieve the effect of improving mixing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
1. Introduction
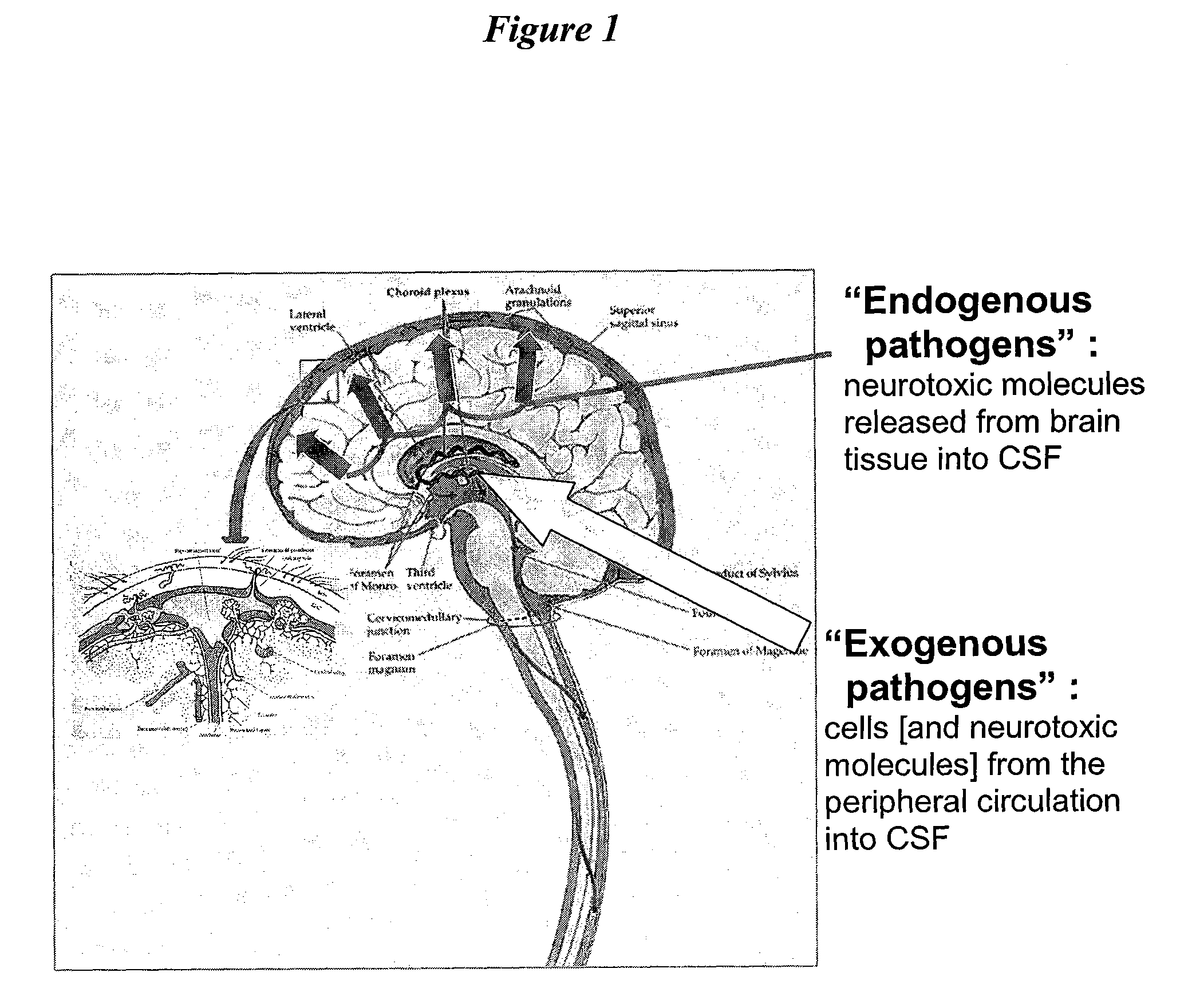
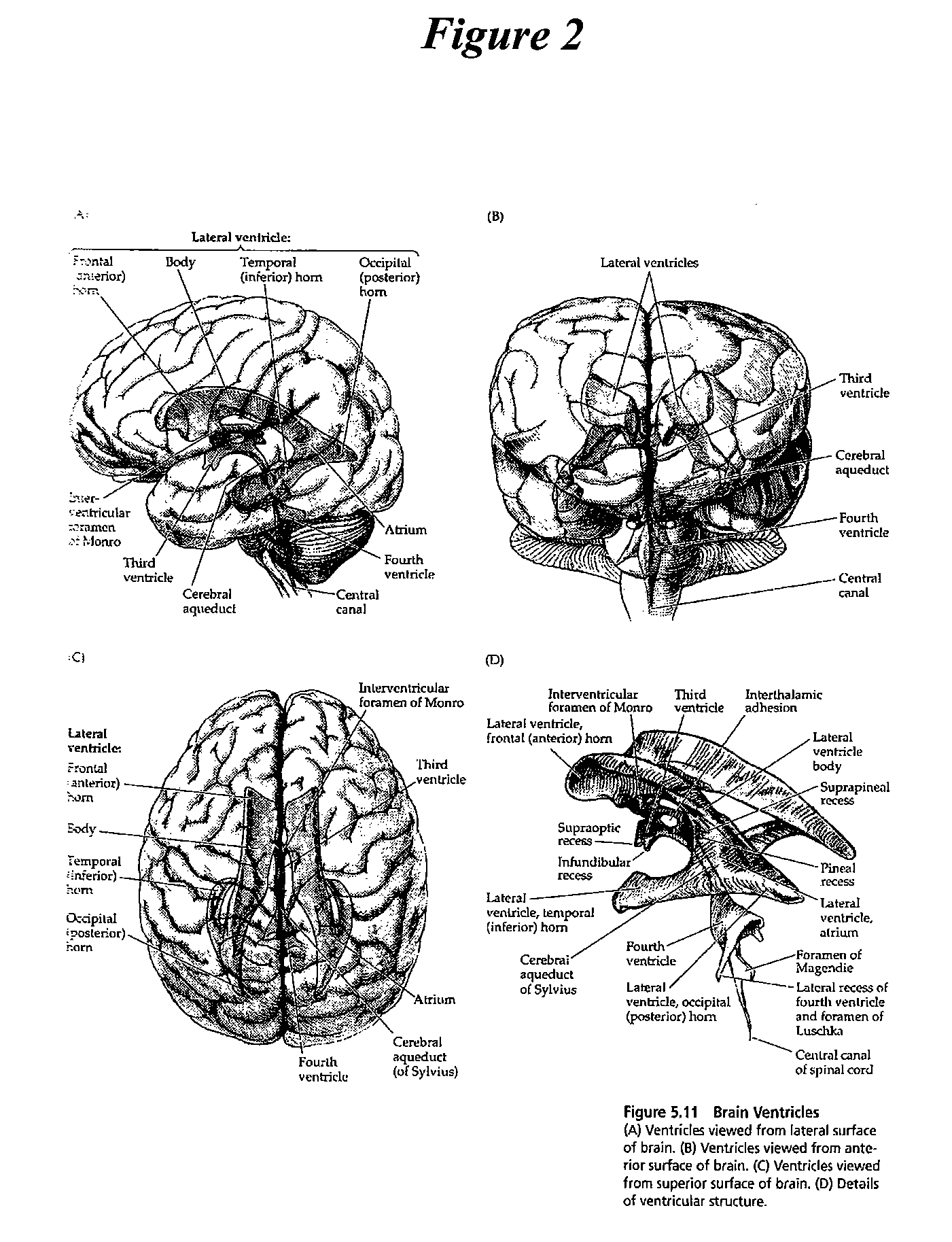
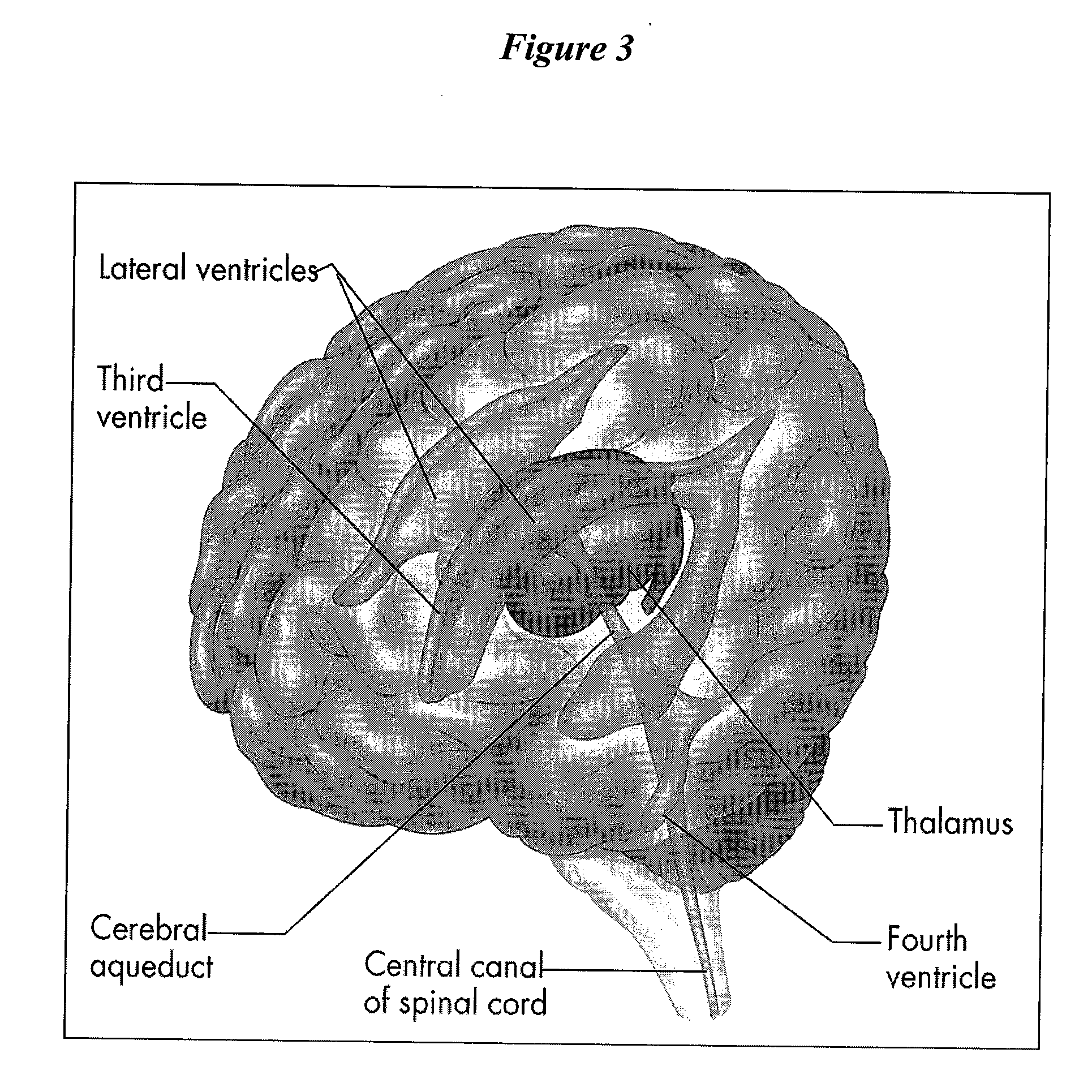
[0087]The present invention provides methods, devices and systems for removing, detecting, returning and delivering compounds from and / or to a patient's cerebrospinal fluid (CSF) space. The removal and / or delivery of specific compounds can be tailored to the pathology of the specific disease. The removal is targeted and specific, for example, through the use of specific size-exclusion thresholds, antibodies against specific toxins, and other chromatographic techniques, as well as delivery and / or removal of targeted therapeutic agents. The invention finds use as a diagnostic, therapeutic and drug delivery platform for a variety of diseases affecting the CNS by accessing the CSF space.
[0088]For the first time, the present invention offers a targeted, focused and logical treatment platform to treat a variety of debilitating and often devastating neurological diseases to which there are presently limited and ineffective treatment options. Exemplified disease conditions tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com