Methods and molecules for modulating an immune response
a technology of immune response and modulation, applied in the direction of antibody medical ingredients, drug compositions, peptides, etc., can solve the problems of asthma symptoms, achieve the effects of reducing the risk of allergic asthma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods for Isolating and Characterizing SHIgM12
[0093]Isolation of human antibodies—Human serum samples were obtained from the dysproteinemia clinic, and those exhibiting an Ig clonal peak of greater than 20 mg / ml were chosen for further evaluation. The selected samples were from 50 patients with a wide variety of conditions characterized by a monoclonal IgM spike, including Waldenstrom's macroglobulinemia, lymphoma, and monoclonal gammopathy of undetermined significance. Sera were dialyzed against water, and precipitates were collected by centrifugation at 14,000 rpm for 30 minutes and dissolved in phosphate buffered saline (PBS). The samples were centrifuged and chromatographed on a Superose-6 column (Amersham Pharmacia, Piscataway, N.J.). IgM fractions were pooled and analyzed by SDS-PAGE, and protein concentrations were determined by reading absorbance at 280 nm. IgM solutions were sterile filtered and cryopreserved. The antibody sHIgM12 was identified based on its...
example 2
A Monoclonal Human IgM Antibody Binds Mouse DC
[0117]DC were obtained in culture following incubation of mouse bone marrow cells in media supplemented with GM-CSF and IL-4. Cells from seven day cultures were incubated with purified antibodies isolated from human sera, and stained with fluoresceinated goat anti-human antibody as well as antibodies specific for cell surface molecules typically expressed on DC. As shown in FIG. 1, the human antibody sHIgM12 bound cells in the cultures that expressed high levels of CD11c, class II, and CD86. Polyclonal human IgM, as well as the other tested monoclonal antibodies from patients with gammopathies or from EBV-transformed cell lines did not appreciably bind the DC populations.
[0118]To determine when the cell surface determinant recognized by the sHIgM12 antibody first appears during the in vitro development of DC, cultured cells were analyzed by flow cytometry at various times during the culture procedure. The determinant first appeared on da...
example 3
The SHIgM12 Antibody Potentiates Dendritic Antigen-Presenting Function
[0120]To determine whether binding of sHIgM12 to the surface of DC influences the pattern of expressed cell surface molecules, day 7 DC cultures were supplemented with 10 μg / ml antibody, incubated overnight, and analyzed by flow cytometry. No changes in cell surface markers specific to sHIgM12 treatment were observed as compared to cultures treated with human polyclonal IgM antibodies or other monoclonal human IgM antibodies.
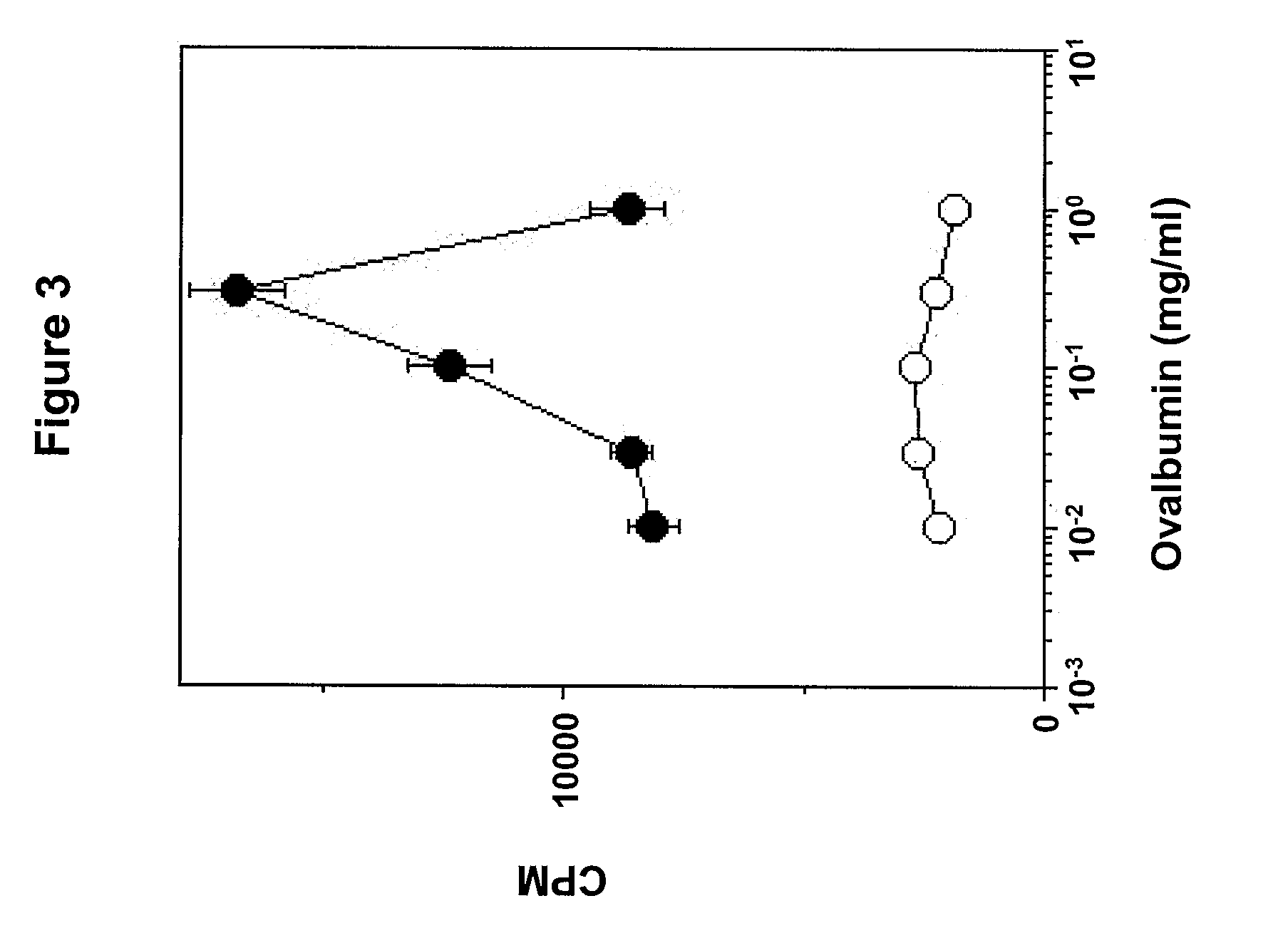
[0121]The antigen-presenting functions of the DC were assessed in vitro. Antibody-treated DC were pulsed with peptide antigen and used to stimulate naïve antigen-specific T cells freshly isolated from OT-1 and DO-11 transgenic mice. T cell activation was measured by incorporation of 3H-thymidine as described in Example 1. DC that were pulsed with a class I-binding peptide (Ser-Ile-Ile-Asn-Phe-Glu-Lys-Leu; SEQ ID NO:1) and incubated with polyclonal HIgM control antibody were able to activate na...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com